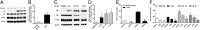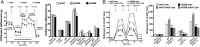Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer - PubMed (original) (raw)
Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer
Csaba Szabo et al. Proc Natl Acad Sci U S A. 2013.
Abstract
The physiological functions of hydrogen sulfide (H2S) include vasorelaxation, stimulation of cellular bioenergetics, and promotion of angiogenesis. Analysis of human colon cancer biopsies and patient-matched normal margin mucosa revealed the selective up-regulation of the H2S-producing enzyme cystathionine-β-synthase (CBS) in colon cancer, resulting in an increased rate of H2S production. Similarly, colon cancer-derived epithelial cell lines (HCT116, HT-29, LoVo) exhibited selective CBS up-regulation and increased H2S production, compared with the nonmalignant colonic mucosa cells, NCM356. CBS localized to the cytosol, as well as the mitochondrial outer membrane. ShRNA-mediated silencing of CBS or its pharmacological inhibition with aminooxyacetic acid reduced HCT116 cell proliferation, migration, and invasion; reduced endothelial cell migration in tumor/endothelial cell cocultures; and suppressed mitochondrial function (oxygen consumption, ATP turnover, and respiratory reserve capacity), as well as glycolysis. Treatment of nude mice with aminooxyacetic acid attenuated the growth of patient-derived colon cancer xenografts and reduced tumor blood flow. Similarly, CBS silencing of the tumor cells decreased xenograft growth and suppressed neovessel density, suggesting a role for endogenous H2S in tumor angiogenesis. In contrast to CBS, silencing of cystathionine-γ-lyase (the expression of which was unchanged in colon cancer) did not affect tumor growth or bioenergetics. In conclusion, H2S produced from CBS serves to (i) maintain colon cancer cellular bioenergetics, thereby supporting tumor growth and proliferation, and (ii) promote angiogenesis and vasorelaxation, consequently providing the tumor with blood and nutritients. The current findings identify CBS-derived H2S as a tumor growth factor and anticancer drug target.
Conflict of interest statement
Conflict of interest statement: C.S. and M.R.H. are founders of and C. Chao is consultant at CBS Therapeutics, a start-up company involved in research and development of cystathionine-β-synthase inhibitors.
Figures
Fig. 1.
CBS is overexpressed in human colorectal cancer. (A) Representative Western blot of CBS, CSE, and 3-MST protein expression in human colorectal cancer specimens, paired with the corresponding normal mucosa tissues. PVDF membranes were probed with rabbit polyclonal antibodies against CBS, CSE, and 3-MST. (B) Densitometric analyses of CBS expression, in seven pairs of human colorectal cancers and the patient-matched normal mucosa, showed an approximately sevenfold increase in CBS protein expression in colon cancer (arbitrary relative densitometric units were normalized with β-actin using image analysis software) (*P < 0.05 vs. normal mucosa). (C and D) CBS was highly expressed in three different colon cancer cell lines (LoVo, HCT116, and HT29); low expression was detected in the nontumorigenic normal colon mucosa cells (NCM356) (arbitrary relative densitometric units were normalized with β-actin using image analysis software) (*P < 0.05 vs. NCM356 cells). H2S production was measured in human colorectal cancer specimens (E) and in colon cancer cell lines (F) by the methylene blue method. H2S production was stimulated in tissue or cell lysates by incubation at 37 °C (30 min) in presence of the CBS substrates
l
-cysteine (3 mM) and
l
-homocysteine (0.5 mM). CBS activity was significantly higher in colon cancer tissues, compared with their corresponding controls. AOAA (1 mM) blocked the H2S-producing activity of CBS in the tissue extracts (*P < 0.05 vs. corresponding from normal mucosa and #P < 0.05 vs. vehicle), whereas PAG (3 mM) had no significant effect. HCT116 cells exhibited the highest rate of H2S production, as measured by the methylene blue method in cell lysates (*P < 0.05 vs. corresponding values in NCM356 and #P < 0.05 vs. vehicle). Western blots show representatives of at least n = 3 experiments; H2S measurements represent mean ± SEM of at least n = 3 determinations.
Fig. 2.
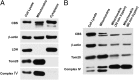
Presence of CBS in cytosolic and mitochondrial fractions of HCT116 cells. (A) CBS was detected in whole cell lysates as well as in mitochondrial and cytoplasmic cell fractions harvested from HCT116 cells. (B) Limited trypsin digestion of isolated mitochondria (30–60 min) reduced mitochondrial CBS, as well as the mitochondrial outer membrane protein Tom20, while enriching complex IV (an inner membrane protein). Each Western blot is representative of at least three independent experiments.
Fig. 3.
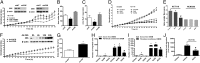
ShRNA mediated down-regulation of CBS or pharmacological inhibition by AOAA inhibits proliferative, migratory, and invading activity of HCT116 cells in vitro. (A and B) The lentiviral shRNA vectors targeting CBS (shCBS) and CSE (shCSE) were transfected into HCT116 cells. A nontargeting sequence was used as control (shNT). The shRNA approach inhibited the expression of both CBS and CSE genes at the protein level, as shown by Western blotting (Inset). Following CBS and CSE silencing, cells were seeded at the density of 3,000 cells per well in xCELLigence plates and proliferation was monitored for 36 h. Down-regulation of CBS, but not CSE, significantly reduced HCT116 proliferation rate (*P < 0.05 vs. shNT). (C) shCBS but not shCSE yielded lower H2S production in cellular homogenates (P < 0.05 vs. shNT). (D and E) HCT116 cells exhibited a significantly higher proliferation rate, compared with NCM356 cells. AOAA (1 mM) did not affect NCM356 growth, but markedly reduced HCT116 cell proliferation (*P < 0.05 vs. NCM356 and #P < 0.05 vs. vehicle), whereas PAG (3 mM) did not affect cell proliferation. (F and G) Adenoviral-mediated CBS overexpression enhances the proliferation rate of NCM356 cells. The NCM356 cells were infected overnight with a CBS expressing adenovirus (Ad-CBS, 10 multicitiplies of infection) or its control, a green fluorescent protein (Ad-GFP). The culture media was then replaced and cells were seeded in XCELLigence plates at 3,000 cells per well. Cell proliferation was then measured in real-time over 36 h. Effective overexpression of CBS was detected within 12–24 h following infection (Inset). Adenoviral-mediated CBS overexpression significantly enhanced NCM356 cell proliferation (*P < 0.05 vs. Ad-GFP). (H and I) The effect of AOAA and NaHS was also tested on HCT116 cell migration (H) and invasion (I). Cells were pretreated with either vehicle or AOAA (1 mM) and seeded in serum-free DMEM (0.1% albumin) in the upper chamber of a Transwell insert uncoated (migration assay) or coated with growth factor reduced matrigel (invasion assay). Migration and invasion were stimulated by fibroblast-conditioned media in the lower chamber for 6 and 24 h, respectively. NaHS (30 µM) in the lower chamber slightly enhanced HCT116 cell migration and invasion in serum-free media. AOAA markedly reduced fibroblast derived growth factor-induced HCT116 cell migration and invasion (*P < 0.05 and #P < 0.05). (J) In a coculture of human endothelial cells (EAhy926) and colon cancer cells (HCT116), HCT116 cells were seeded in the lower chamber of a Transwell insert and cultured to confluence. Endothelial cells were then seeded into the upper chamber in serum-free DMEM and allowed to migrate for 4 h at 37 °C. The presence of HCT116 in the lower chamber markedly increased the number of migrated endothelial cells, and this effect was reduced by AOAA (*P < 0.05). Western blots are representative of at least three independent experiments; proliferation/migration/invasion assays and H2S measurements represent mean ± SEM of n = 3 determinations.
Fig. 4.
ShRNA-mediated down-regulation of CBS suppresses cellular bioenergetics in HCT116 cells. (A) Oxygen consumption rate (OCR) in HCT116 cells subjected to either nontargeting (shNT, control) or stable lentiviral silencing of CBS or CSE (shCBS, shCSE). shCBS enzyme significantly decreased basal OCR, calculated ATP production, maximal respiration, and spare respiratory capacity (*P < 0.05 or **P < 0.01 vs. shNT), whereas CSE silencing had no effect on the bioenergetic profile. (B) Coupling experiments show that
l
-cysteine (30 nM) elevates OCR in state 3 and state 3u respiration in mitochondria isolated from control shNT cells (*P < 0.05), but not in mitochondria isolated from shCBS cells (#P < 0.05 or ##P < 0.01). Data represent mean ± SEM of n = 4–5 determinations.
Fig. 5.
ShRNA-mediated down-regulation of CBS or pharmacological inhibition by AOAA (9 mg/kg·d−1) inhibits colon cancer growth and tumor angiogenesis in vivo. Effects of shRNA-mediated gene silencing of CBS (shCBS) and CSE (shCSE) on HCT116 tumor xenograft: (A) growth rate (shNT = nontargeting shRNA control), (B) tumor volume at harvest (*P = 0.04), and (C) plasma levels of H2S. (D) Quantification of the effects of CBS silencing on CD31-positive blood vessel density in HCT116 tumor xenografts (*P < 0.0001). (E) Photomicrographs of representative sections (10 μm) from control (shNT) and CBS knockdown (shCBS) xenografts showing CD31-positive blood vessels (brown). Note the increased density of blood vessels in shNT vs. shCBS. Arrows indicate larger vessels and bracket indicates areas of necrosis with shCBS xenograft. Effects of AOAA or vehicle (PBS) on HCT116 tumor xenografts: (F) growth rate, (G) tumor volume (*P = 0.02), (H) wet weight (*P = 0.001), and (I) plasma concentrations of H2S (*P = 0.0005). (J) Photomicrographs of H&E stained formalin-fixed paraffin-embedded sections (5 μm) of the primary colon adenocarcinoma from a patient with stage III disease and Kras mutation (PT), and the corresponding patient-derived tumor xenograft (PDTX). Note the similar morphology of both specimens. (K) Western blot comparing the relative levels of expression of CBS in tissue extracts from the patient’s tumor (T) shown in J and adjacent normal (N) mucosa. (L and M) Effects of AOAA and PBS treatment of growth rates of PDTXs from patient 1 and patient 2, respectively. (N) Summary data from two independent experiments showing the effects of AOAA and PBS on the change in PDTX volume over a 7-d course of treatment (*P = 0.07). Photomicrographs of histological sections are representative of at least n = 6 sections; tumor volume/weight data and H2S measurements represent mean ± SEM of tumors/plasma values obtained from n = 6 mice.
Fig. 6.
l
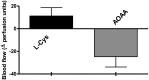
-cysteine increases, whereas AOAA reduces microvessel blood flow in tumor-bearing mice. Tumor-bearing mice were anesthetized by i.p. injections of ketamine-xylazine and a laser Doppler (PeriFlux system 5000) was placed on top of the tumor for microvessel blood flow measurements. After a stabilization period,
l
-cysteine or AOAA were injected s.c. in proximity of the tumor. AOAA caused a marked decrease of the skin microvessel blood flow. Data represent mean ± SEM of n = 6 determinations.
Fig. 7.
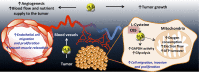
Proposed mechanisms for H2S induced colon cancer growth. As a result of the CBS overexpression, H2S is overproduced in colon cancer cells. H2S serves as an inorganic electron donor, stimulating mitochondrial electron transport, increasing ATP turnover. In addition, it increases the glycolytic activity of the tumor cell. Via these autocrine bioenergetic effects, H2S stimulates cancer cell proliferation, migration, and invasion. In addition, H2S diffuses into the surrounding cells and tissues, stimulating angiogenesis, as well as acting as a vascular relaxant. Via these paracrine effects, H2S promotes the supply of blood and nutrients to the tumor.
Comment in
- Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation.
Szabo C, Hellmich MR. Szabo C, et al. Cell Cycle. 2013 Sep 15;12(18):2915-6. doi: 10.4161/cc.26064. Epub 2013 Aug 21. Cell Cycle. 2013. PMID: 23974103 Free PMC article. No abstract available.
Similar articles
- Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro.
Módis K, Coletta C, Asimakopoulou A, Szczesny B, Chao C, Papapetropoulos A, Hellmich MR, Szabo C. Módis K, et al. Nitric Oxide. 2014 Sep 15;41:146-56. doi: 10.1016/j.niox.2014.03.001. Epub 2014 Mar 22. Nitric Oxide. 2014. PMID: 24667534 Free PMC article. - Screening of a composite library of clinically used drugs and well-characterized pharmacological compounds for cystathionine β-synthase inhibition identifies benserazide as a drug potentially suitable for repurposing for the experimental therapy of colon cancer.
Druzhyna N, Szczesny B, Olah G, Módis K, Asimakopoulou A, Pavlidou A, Szoleczky P, Gerö D, Yanagi K, Törö G, López-García I, Myrianthopoulos V, Mikros E, Zatarain JR, Chao C, Papapetropoulos A, Hellmich MR, Szabo C. Druzhyna N, et al. Pharmacol Res. 2016 Nov;113(Pt A):18-37. doi: 10.1016/j.phrs.2016.08.016. Epub 2016 Aug 10. Pharmacol Res. 2016. PMID: 27521834 Free PMC article. - Regulation of CyR61 expression and release by 3-mercaptopyruvate sulfurtransferase in colon cancer cells.
Ascenção K, Lheimeur B, Szabo C. Ascenção K, et al. Redox Biol. 2022 Oct;56:102466. doi: 10.1016/j.redox.2022.102466. Epub 2022 Sep 8. Redox Biol. 2022. PMID: 36113340 Free PMC article. - The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer.
Hellmich MR, Coletta C, Chao C, Szabo C. Hellmich MR, et al. Antioxid Redox Signal. 2015 Feb 10;22(5):424-48. doi: 10.1089/ars.2014.5933. Epub 2014 Jun 20. Antioxid Redox Signal. 2015. PMID: 24730679 Free PMC article. Review. - Emerging roles of cystathionine β-synthase in various forms of cancer.
Ascenção K, Szabo C. Ascenção K, et al. Redox Biol. 2022 Jul;53:102331. doi: 10.1016/j.redox.2022.102331. Epub 2022 May 10. Redox Biol. 2022. PMID: 35618601 Free PMC article. Review.
Cited by
- Engineering of an endogenous hydrogen sulfide responsive smart agent for photoacoustic imaging-guided combination of photothermal therapy and chemotherapy for colon cancer.
Tian Q, Wang X, Song S, An L, Yang S, Huang G. Tian Q, et al. J Adv Res. 2022 Nov;41:159-168. doi: 10.1016/j.jare.2022.01.018. Epub 2022 Feb 9. J Adv Res. 2022. PMID: 36328745 Free PMC article. - Tadalafil Integrates Nitric Oxide-Hydrogen Sulfide Signaling to Inhibit High Glucose-induced Matrix Protein Synthesis in Podocytes.
Lee HJ, Feliers D, Mariappan MM, Sataranatarajan K, Choudhury GG, Gorin Y, Kasinath BS. Lee HJ, et al. J Biol Chem. 2015 May 8;290(19):12014-26. doi: 10.1074/jbc.M114.615377. Epub 2015 Mar 9. J Biol Chem. 2015. PMID: 25752605 Free PMC article. - Cystathionine-β-synthase expression correlates with tumour progression and adverse prognosis in patients with colon cancer.
Hu XJ, Sun Y, Liu GJ, Zhang J, Zhang LX, Peng YH. Hu XJ, et al. J Int Med Res. 2024 Sep;52(9):3000605241263726. doi: 10.1177/03000605241263726. J Int Med Res. 2024. PMID: 39324183 Free PMC article. - Gasotransmitters in Gametogenesis and Early Development: Holy Trinity for Assisted Reproductive Technology-A Review.
Nevoral J, Bodart JF, Petr J. Nevoral J, et al. Oxid Med Cell Longev. 2016;2016:1730750. doi: 10.1155/2016/1730750. Epub 2016 Aug 8. Oxid Med Cell Longev. 2016. PMID: 27579148 Free PMC article. Review. - Recent therapeutic approaches to cystathionine beta-synthase-deficient homocystinuria.
Majtan T, Kožich V, Kruger WD. Majtan T, et al. Br J Pharmacol. 2023 Feb;180(3):264-278. doi: 10.1111/bph.15991. Epub 2022 Dec 8. Br J Pharmacol. 2023. PMID: 36417581 Free PMC article. Review.
References
- Cai WJ, et al. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76(1):29–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources