Local palmitoylation cycles define activity-regulated postsynaptic subdomains - PubMed (original) (raw)
Local palmitoylation cycles define activity-regulated postsynaptic subdomains
Yuko Fukata et al. J Cell Biol. 2013.
Abstract
Distinct PSD-95 clusters are primary landmarks of postsynaptic densities (PSDs), which are specialized membrane regions for synapses. However, the mechanism that defines the locations of PSD-95 clusters and whether or how they are reorganized inside individual dendritic spines remains controversial. Because palmitoylation regulates PSD-95 membrane targeting, we combined a conformation-specific recombinant antibody against palmitoylated PSD-95 with live-cell super-resolution imaging and discovered subsynaptic nanodomains composed of palmitoylated PSD-95 that serve as elementary units of the PSD. PSD-95 in nanodomains underwent continuous de/repalmitoylation cycles driven by local palmitoylating activity, ensuring the maintenance of compartmentalized PSD-95 clusters within individual spines. Plasma membrane targeting of DHHC2 palmitoyltransferase rapidly recruited PSD-95 to the plasma membrane and proved essential for postsynaptic nanodomain formation. Furthermore, changes in synaptic activity rapidly reorganized PSD-95 nano-architecture through plasma membrane-inserted DHHC2. Thus, the first genetically encoded antibody sensitive to palmitoylation reveals an instructive role of local palmitoylation machinery in creating activity-responsive PSD-95 nanodomains, contributing to the PSD (re)organization.
Figures
Figure 1.
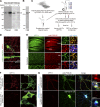
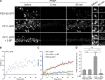
Selection of PF11, a recombinant antibody specific for palmitoylated PSD-95. (A) PSD-95-GFP-PSTCD metabolically labeled with [3H]palmitic acid in HEK293T cells was analyzed by fluorography ([3H]palm) and Coomassie brilliant blue (CBB) staining. Closed and open arrowheads indicate the positions of palmitoylated and nonpalmitoylated PSD-95, respectively. (B) Palmitoylated PSD-95-GFP-PSTCD was purified to near homogeneity. (C) A combinatorial recombinant antibody library was screened in vitro against palmitoylated PSD-95. A promising clone, PF11, was obtained. (D) Indirect PF11 immunofluorescence. The signal (hPF11, red) disappeared in PSD-95 knockdown neurons (shPSD-95, green, arrows). Bars, 20 µm (5 µm, magnified). (E) hPF11 (green) and PSD-95 (red) antibodies showed the similar staining pattern in the hippocampus (top) and cerebellum (bottom) of adult mouse brain sections. Glutamatergic presynapses were labeled with the mixture of vGlut1 and vGlut2 antibodies (blue). DG, dentate gyrus; Mo, molecular layer; PC, Purkinje cell layer; Gr, granule cell layer. Regions in the molecular layer of DG and cerebellum are magnified (right panels). Bars: (top) 500 µm; (bottom left) 100 µm; (bottom right) 2.5 µm. (F) HEK293T cells were cotransfected with DHHC2 and either PSD-95 wild-type (WT) or cysteine-mutated palmitoylation-deficient PSD-95 CS, and cells were stained with hPF11 (red). Bar, 10 µm. (G) hPF11 staining (red) was detected in neurons expressing shPSD-95 (marked by GFP, blue pseudocolor) and complemented by shRNA-resistant (res) PSD-95 WT (stained by anti-PSD-95, green pseudocolor), but not by palmitoylation-deficient resPSD-95 CS. Bars, 10 µm (1 µm, dendritic spines magnified).
Figure 2.
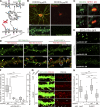
PF11 distinguishes postsynaptic PSD-95 from extrasynaptic PSD-95. (A and B) Hippocampal neurons were stained triply with hPF11, PSD-95, and vGlut1 antibodies. Asterisks in intensity profiles (B) indicate postsynaptic hPF11 signals (green) colocalized with total PSD-95 (red) and vGlut1 staining (blue), and arrows indicate extrasynaptic PSD-95 signals devoid of hPF11 and vGlut1 signals. A dendritic region indicated by a thick black bar in the intensity profile is magnified (B). Representative profiles from ten repeats (three neurons) are shown (B). (C and D) Hippocampal neurons were treated with or without 2-bromopalmitate (2-BP; 100 µM, 6 h) and stained with hPF11 and PSD-95 antibodies. Representative dendritic regions are shown (C). 300–400 dendritic clusters and 30–40 soma clusters from 5 neurons were analyzed for fluorescence intensities of green and red channels (D). palm, palmitoylated PSD-95. n.s., not significant; ***, P < 0.001, determined by one-way ANOVA with post-hoc Tukey’s test. Bars: (A) 10 µm; (B) 1 µm; (C) 5 µm.
Figure 3.
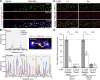
PF11 as a conformation-specific intrabody for palmitoylated PSD-95. (A–D) In the presence of DHHC2, PSD-95-mCherry and PF11-GFP were colocalized near the plasma membrane and on intracellular vesicular structures in HEK293T cells (B and C, white arrowheads). Treatment with 2-BP (100 µM, 8 h) dissociated PF11-GFP from the plasma membrane (asterisks) and intracellular aggregates of PSD-95-mCherry (D, black arrowheads). Bars, 10 µm (2 µm, magnified in C). (E) Immunoprecipitation (IP) of PF11-GFP from HEK293T cells. IB, immunoblotting. CC7, unrelated recombinant antibody; closed arrow, PF11-GFP; open arrow, CC7-GFP; closed arrowhead, PSD-95-mCherry. (F) PSD-95 constructs to test the specificity of PF11 binding using methods in Fig. 3, B and/or E. GuK, guanylate kinase domain; S, serine residues replacing palmitoyl cysteines. Red chain, palmitoyl; green, prenyl; blue, myristoyl groups. tag, mCherry or GFP. (G) Blockade of palmitoylation with 2-BP (100 µM, 18 h) dispersed synaptic labeling by PF11-GFP. Red, mCherry as a fill-in marker. Arrows, multiple subspine clusters. Bars, 5 µm (2 µm, magnified). (H) PF11-GFP tracked a synaptic increase of palmitoylated PSD-95 upon TTX treatment. Arrowheads, multicluster spines. Bar, 5 µm. (I) Immunoprecipitation of PF11-GFP from hippocampal neurons treated with 2-BP or TTX. Arrow, PF11-GFP. Closed and open arrowheads are as in Fig. 1 A.
Figure 4.
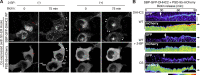
Novel PSD-95 nanodomains as building blocks of postsynaptic membrane regions. (A and B) Live-cell imaging of PF11-GFP by STED microscopy (green), but not by conventional confocal microscopy (red pseudocolor), efficiently detects multiple subspine clusters (1 to 4 clusters/spine) in neurons. Bars: (A) 1 µm; (B) 500 nm. (C and D) Size of subsynaptic nanodomains. Subsynaptic clusters of PF11-GFP and PSD-95-GFP visualized by live STED imaging were measured across the longest axis. Approximately 200 clusters were randomly selected from 10 live-imaging experiments. FWHM, full width at half maximum. ***, P < 0.001 by Student’s t test. (E) The size of postsynaptic membrane regions (determined by confocal microscopy, red in B, according to diameter at the longest axis) correlates well with the number of PF11-labeled subsynaptic nanodomains (determined by STED, green in B). 130 postsynapses from 5 live neurons were analyzed. (F) Dual-color STED (2C-STED) analysis of fixed neurons. Postsynaptic hPF11 labeling (red pseudocolor) shows nanodomain structures (arrowheads), overlapping with total PSD-95 labeling (green pseudocolor, top panels) and apposed to presynaptic vGlut1 labeling (green pseudocolor, bottom). The same fields were sequentially imaged in the confocal mode. Bars, 500 nm. (G) The size of PF11-labeled postsynaptic regions was measured by STED microscopy, as described in
Fig. S2 B
for a representative image from F, and the frequency histogram is shown. Peaks by the Gaussian approximation centered at 225, 420, and 625 nm are shown in red dashed lines. In total, 450 clusters from 6 neurons (three independent experiments) were analyzed. Representative regions with one, two, and three nanodomains are shown (insets). Bars, 200 nm. (H) Individual nanodomains (red pseudocolor) in a spine are associated with AMPARs (green pseudocolor). sGluA1, surface-expressed GluA1. Arrows, nanodomains labeled by PF11-Venus. Bar, 500 nm.
Figure 5.
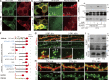
Continuous palmitate cycling on PSD-95 nanodomains is mediated by local palmitoylating activity. (A) Dendritic regions of transfected hippocampal neurons were bleached, and then the fluorescent recovery of PSD-95-GFP or PF11-GFP at the individual clusters was monitored. Right, representative magnified images before bleaching and at 60 min after bleaching. Note that the recovered PF11-GFP kept its initially distinguished nanodomain locations (arrowheads). Asterisks, diffuse fluorescent signals of PF11-GFP in dendritic shaft that were subtracted from the FRAP at the clusters. Bars: (left) 5 µm; (right) 0.5 µm. (B) Sample FRAP of PF11-GFP at subsynaptic clusters. (C) Average FRAP of PF11-GFP (red), PF11-GFP treated with 2-BP (green), and PSD-95-GFP (blue). n = 3 experiments (5–6 clusters/experiment). (D) Fluorescence recovery of PSD-95-GFP and PF11-GFP at 60 min after bleaching in the presence or absence of 2-BP. ***, P < 0.001 by one-way ANOVA with post-hoc Tukey’s test (n = 3 experiments [5–6 clusters/experiment]).
Figure 6.
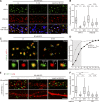
Surface-expressed DHHC2 is essential for generation of postsynaptic nanodomains. (A) DHHC2 constructs used. EKKNR, an ER-retention signal; SEP, super-ecliptic pHluorin. Blue boxes, DHHC catalytic domain. extra, extracellular space; cyto, cytoplasm; lum, lumen. (B) Surface-expressed and total DHHC-HA are shown in green and red, respectively. Bars: (top) 10 µm; (bottom) 2 µm. (C) Endogenous DHHC2 (green) exists as small discrete clusters (arrows) at the center of hPF11-stained postsynaptic regions (red) in a spine. Bar, 1 µm. (D) Endosomal (arrowheads) and synaptic membrane localization (arrows) of DHHC2. GFP-DHHC2 or DHHC2-SEP was coexpressed with TfR-mCherry or PSD-95-mCherry. Bar, 5 µm. (E and F) Hippocampal neurons were transfected with PF11-GFP-miR-DHHC2 (for co-cistronic expression) and miRNA-resistant DHHC2 (resDHHC2-WT, CS, or ER), and live-imaged using STED microscopy. PF11-GFP–labeled nanodomains (gray boxes) and multi-nanodomain synapses (E, arrowheads; white boxes) were counted (F). In total, 35 (WT), 4 (CS), and 11 (ER) dendrites from two or three independent experiments were analyzed. Bar: (E) 1 µm. (G and H) miRNA against DHHC2 (marked by EmGFP) was expressed with resDHHC2 WT or –ER. Neurons were stained using a conventional PSD-95 antibody (red) and imaged by confocal microscopy. miLacZ indicates a control miRNA. Arrows denote PSD-95-negative dendritic spines/filopodia (G). Approximately 50 dendrites from 2 independent experiments were analyzed (H). resDH2, resDHHC2. ***, P < 0.001, by one-way ANOVA with post-hoc Tukey’s test (F and H). Bar: (G) 5 µm.
Figure 7.
DHHC2 directly nucleates PSD-95 assembly at the plasma membrane through local palmitoylation. (A) HEK293T cells were cotransfected with a bi-cistronic RUSH vector containing streptavidin-Ii (Str-ER Hook) and streptavidin-binding peptide (SBP)-GFP-DHHC2 as well as PSD-95-mCherry. Synchronized release of DHHC2 from the ER was induced by the addition of biotin with or without 2-BP. Arrowheads denote signals at the plasma membrane. Bar, 10 µm. (B) Kymograph analysis. The fluorescence intensities of GFP and mCherry were measured along red lines in A. White arrows indicate the timing when DHHC2 arrived at the plasma membrane. Black arrows indicate the position of the plasma membrane (at 90 min). CS, inactive DHHC2. Bar, 2.5 µm.
Figure 8.
Changes in synaptic activity remodel postsynaptic PSD-95 nanodomains through local DHHC2 activity. (A and B) Neurons were treated with 90 mM KCl for 5 min and recovered for 60 min in the basal medium (wash out, WO). Neurons were stained triply with hPF11 (green), PSD-95 (red), and vGlut1 (blue) antibodies, and the confocal fluorescence intensities of green and red channels at PSD-95–positive clusters were measured. In total, 170–240 clusters from three neurons were analyzed. ***, P < 0.001 by one-way ANOVA with post-hoc Tukey’s test (B). Bar, 5 µm. (C and D) Neurons treated with high K+ were analyzed by 2C-STED imaging of PSD-95 (green pseudocolor) and hPF11 (red pseudocolor), and the distance between the peaks with the highest intensity of hPF11 (arrowheads) and PSD-95 (arrows) clusters was measured (as described in
Fig. S5 A
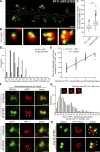
). The brightness of magnified images with KCl treatment is enhanced. Gray region in D indicates the subresolution range for STED imaging. In total, 150–200 clusters from 10 neurons (two independent experiments) were analyzed. ***, P < 0.001 by Student’s t test (D). Bars, 500 nm (200 nm, magnified). (E and F) Neurons were cotransfected with miR-DHHC2 and HA-resDHHC2-WT or ER (red), and treated as in (A). The confocal fluorescence intensity of hPF11 (green) overlapped with vGlut1 (not depicted) was measured. In total, 230–420 clusters from 8 neurons (three independent experiments) were analyzed. ***, P < 0.001 by one-way ANOVA with post-hoc Tukey’s test. Bar, 5 µm.
Figure 9.
Synaptic activity regulates the association of AMPARs with postsynaptic nanodomains. (A and B) Neurons were treated with 90 mM KCl for 5 min and recovered for 60 min. Neurons were analyzed by 2C-STED imaging of surface GluA1 (A, green pseudocolor) and hPF11 (A, red pseudocolor), and the maximum intensity of surface AMPARs on PF11-labeled nanodomains was measured (B). In total, ∼1,000 clusters from 8–12 neurons (two independent experiments) were analyzed. ***, P < 0.001 by one-way ANOVA with post-hoc Tukey’s test. Bar, 500 nm. (C) A DHHC2-mediated subdomain model for PSD (re)organization: organization of nanodomains through local palmitoylation/depalmitoylation cycles. See Discussion.
Similar articles
- Identification of PSD-95 Depalmitoylating Enzymes.
Yokoi N, Fukata Y, Sekiya A, Murakami T, Kobayashi K, Fukata M. Yokoi N, et al. J Neurosci. 2016 Jun 15;36(24):6431-44. doi: 10.1523/JNEUROSCI.0419-16.2016. J Neurosci. 2016. PMID: 27307232 Free PMC article. - Palmitoylation regulates glutamate receptor distributions in postsynaptic densities through control of PSD95 conformation and orientation.
Jeyifous O, Lin EI, Chen X, Antinone SE, Mastro R, Drisdel R, Reese TS, Green WN. Jeyifous O, et al. Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):E8482-E8491. doi: 10.1073/pnas.1612963113. Epub 2016 Dec 12. Proc Natl Acad Sci U S A. 2016. PMID: 27956638 Free PMC article. - S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95.
Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, Snyder SH. Ho GP, et al. Neuron. 2011 Jul 14;71(1):131-41. doi: 10.1016/j.neuron.2011.05.033. Neuron. 2011. PMID: 21745643 Free PMC article. - Postsynaptic nanodomains generated by local palmitoylation cycles.
Fukata M, Sekiya A, Murakami T, Yokoi N, Fukata Y. Fukata M, et al. Biochem Soc Trans. 2015 Apr;43(2):199-204. doi: 10.1042/BST20140238. Biochem Soc Trans. 2015. PMID: 25849917 Review. - Posttranslational Modifications Regulate the Postsynaptic Localization of PSD-95.
Vallejo D, Codocedo JF, Inestrosa NC. Vallejo D, et al. Mol Neurobiol. 2017 Apr;54(3):1759-1776. doi: 10.1007/s12035-016-9745-1. Epub 2016 Feb 16. Mol Neurobiol. 2017. PMID: 26884267 Review.
Cited by
- ELMOD2 is anchored to lipid droplets by palmitoylation and regulates adipocyte triglyceride lipase recruitment.
Suzuki M, Murakami T, Cheng J, Kano H, Fukata M, Fujimoto T. Suzuki M, et al. Mol Biol Cell. 2015 Jun 15;26(12):2333-42. doi: 10.1091/mbc.E14-11-1504. Epub 2015 Apr 22. Mol Biol Cell. 2015. PMID: 25904333 Free PMC article. - Robust nanoscopy of a synaptic protein in living mice by organic-fluorophore labeling.
Masch JM, Steffens H, Fischer J, Engelhardt J, Hubrich J, Keller-Findeisen J, D'Este E, Urban NT, Grant SGN, Sahl SJ, Kamin D, Hell SW. Masch JM, et al. Proc Natl Acad Sci U S A. 2018 Aug 21;115(34):E8047-E8056. doi: 10.1073/pnas.1807104115. Epub 2018 Aug 6. Proc Natl Acad Sci U S A. 2018. PMID: 30082388 Free PMC article. - AMPAR Palmitoylation Tunes Synaptic Strength: Implications for Synaptic Plasticity and Disease.
Koster KP. Koster KP. J Neurosci. 2019 Jun 26;39(26):5040-5043. doi: 10.1523/JNEUROSCI.0055-19.2019. J Neurosci. 2019. PMID: 31243093 Free PMC article. No abstract available. - Role of Palmitoylation of Postsynaptic Proteins in Promoting Synaptic Plasticity.
Matt L, Kim K, Chowdhury D, Hell JW. Matt L, et al. Front Mol Neurosci. 2019 Jan 31;12:8. doi: 10.3389/fnmol.2019.00008. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30766476 Free PMC article. Review. - Unraveling the mysteries of dendritic spine dynamics: Five key principles shaping memory and cognition.
Kasai H. Kasai H. Proc Jpn Acad Ser B Phys Biol Sci. 2023;99(8):254-305. doi: 10.2183/pjab.99.018. Proc Jpn Acad Ser B Phys Biol Sci. 2023. PMID: 37821392 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases