Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer - PubMed (original) (raw)
doi: 10.1136/gutjnl-2013-304739. Epub 2013 Jul 11.
Melissa K Friswell, Abdullah Alswied, Carol L Roberts, Fei Song, Paul K Flanagan, Paul Knight, Caroline Codling, Julian R Marchesi, Craig Winstanley, Neil Hall, Jonathan M Rhodes, Barry J Campbell
Affiliations
- PMID: 23846483
- PMCID: PMC3995253
- DOI: 10.1136/gutjnl-2013-304739
Free PMC article
Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer
Maelle Prorok-Hamon et al. Gut. 2014 May.
Free PMC article
Abstract
Objective: Colonic mucosa-associated Escherichia coli are increased in Crohn's disease (CD) and colorectal cancer (CRC). They variously haemagglutinate, invade epithelial cell lines, replicate within macrophages, translocate across M (microfold) cells and damage DNA. We investigated genes responsible for these effects and their co-association in colonic mucosal isolates.
Design: A fosmid library yielding 968 clones was prepared in E coli EPI300-T1 using DNA from a haemagglutinating CRC isolate, and resulting haemagglutinating clones were 454-pyrosequenced. PCR screening was performed on 281 colonic E coli isolates from inflammatory bowel disease (IBD) (35 patients), CRC (21) and controls (24; sporadic polyps or irritable bowel syndrome).
Results: 454-Pyrosequencing of fosmids from the haemagglutinating clones (n=8) identified the afimbrial adhesin afa-1 operon. Transfection of afa-1 into E coli K-12 predictably conferred diffuse adherence plus invasion of HEp-2 and I-407 epithelial cells, and upregulation of vascular endothelial growth factor. E coli expressing afaC were common in CRC (14/21, p=0.0009) and CD (9/14, p=0.005) but not ulcerative colitis (UC; 8/21) compared with controls (4/24). E coli expressing both afaC and lpfA (relevant to M-cell translocation) were common in CD (8/14, p=0.0019) and CRC (14/21, p=0.0001), but not UC (6/21) compared with controls (2/24). E coli expressing both afaC and pks (genotoxic) were common in CRC (11/21, p=0.0015) and UC (8/21, p=0.022), but not CD (4/14) compared with controls (2/24). All isolates expressed dsbA and htrA relevant to intra-macrophage replication, and 242/281 expressed fimH encoding type-1 fimbrial adhesin.
Conclusions: IBD and CRC commonly have colonic mucosal E coli that express genes that confer properties relevant to pathogenesis including M-cell translocation, angiogenesis and genotoxicity.
Keywords: BACTERIAL ADHERENCE; BACTERIAL INTERACTIONS; BACTERIAL PATHOGENESIS; E. COLI; GUT INFLAMMATION.
Figures
Figure 1
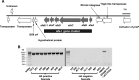
Haemagglutinin-positive E coli in the fosmid clone library derived from E coli HM358 share a common region containing the afimbrial adhesin afa-1 operon. (A) Genomic organisation of the common region shared by haemagglutination (HA)-positive clones includes afaA (encoding a transciptional regulator); afaB (chaperone); afaC (usher); afaD (invasin); draP (linker element) and afaE-1 (an adhesin). (B) PCR for afaC. A 672 bp fragment is detected in all eight haemagglutination-positive clones and E coli HM358. E coli EPI300-T1/pCC1FOS and eight haemagglutination-negative fosmids lacked afaC.
Figure 2
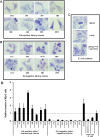
The presence of the afa-1 operon in haemagglutinating E coli correlates with diffuse adherence and invasion to HEp-2 epithelial cells. Giemsa stain of HEp-2 cells infected with E coli strains. (A) All eight haemagglutinin-positive library clones showed diffuse adherence to cell cultures. (B) Eight haemagglutination (HA)-negative fosmid clones chosen at random from the library were non-adherent. (C) Colonic mucosally associated E coli HM358 exhibited a diffusely adherent pattern as per diffusely adherent E coli C1845. The E coli K12 plating strain EPI300T1 containing pCC1Fos was non-adherent. (D) The eight haemagglutination-positive fosmid library clones possessing the afa-1 gene cluster, exhibiting diffuse adherence, showed increased ability to invade Hep-2 cells compared to haemagglutination-negative clones. Invasion calculated as percentage of the original inoculums (multiplicity of infection 10) and expressed relative to E coli LF82 previously shown to be invasive in this cell line. * p<0.05 and *** p<0.001 when compared to the non-invasive plating strain EPI300-T1 containing pCC1Fos alone (mean±SEM; N=3 experiments, each performed with n=3 replicates; Kruskal–Wallis).
Figure 3
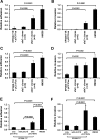
The presence of _afa_-1 in haemagglutinating E coli correlates with ability to adhere to and invade intestinal epithelial cells. Relative ability of eight haemagglutination-positive library clones possessing afa-1 (_afaC_+ as determined by PCR) and haemagglutination-negative clones (n=8) to (A) adhere to and (B) invade I-407 cells. Data mean (±SEM) relative to that observed by E coli HM358; n=4. Increased (C) adherence to, and (D) invasion of differentiated Caco2 cells by _afa-1_-possessing clones was observed. (E and F) E coli EPI300-T1/pCC1FOS transformed with afa-1 (pUCAfa) adheres to and invades I-407 cells. Data expressed relative to plating strain; N=3, each n=3–5 replicates). p Values determined by Kruskal–Wallis. Afa, afimbrial adhesin; afa-1, afimbrial adhesin operon 1; afaC, gene encoding afimbrial adhesin outer membrane usher protein.
Figure 4
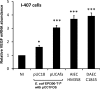
The presence of afa in E coli upregulates vascular endothelial growth factor (VEGF) expression in cultured intestinal epithelial cells. Confluent serum-starved I-407 cells (8×105 cells/well) were infected for 4 h with either wild-type colorectal cancer adherent, invasive E coli HM358, Afa/Dr diffusely adhering E coli C1845 or E coli EPI300-T1/pCC1FOS transformed with afa-1 operon (pUCAfa) or vector alone (pUC18) and total RNA extracted. VEGF mRNA measured by quantitative PCR relative to β actin. Data mean (±SEM) relative to non-infected cells (set at 100%); N=2–4; each n=3 replicates). *p<0.05, *** p<0.001 determined by Kruskal–Wallis. AIEC, adherent, invasive E coli; DAEC, diffusely adherent E coli.
Similar articles
- The prevalence of mucosa-associated diffusely adherent Escherichia coli in children with inflammatory bowel disease.
Walczuk U, Sobieszczańska B, Turniak M, Rzeszutko M, Duda-Madej A, Iwańczak B. Walczuk U, et al. Adv Clin Exp Med. 2019 Jul;28(7):899-905. doi: 10.17219/acem/94149. Adv Clin Exp Med. 2019. PMID: 31066244 - Point mutations in FimH adhesin of Crohn's disease-associated adherent-invasive Escherichia coli enhance intestinal inflammatory response.
Dreux N, Denizot J, Martinez-Medina M, Mellmann A, Billig M, Kisiela D, Chattopadhyay S, Sokurenko E, Neut C, Gower-Rousseau C, Colombel JF, Bonnet R, Darfeuille-Michaud A, Barnich N. Dreux N, et al. PLoS Pathog. 2013 Jan;9(1):e1003141. doi: 10.1371/journal.ppat.1003141. Epub 2013 Jan 24. PLoS Pathog. 2013. PMID: 23358328 Free PMC article. - Microevolution in fimH gene of mucosa-associated Escherichia coli strains isolated from pediatric patients with inflammatory bowel disease.
Iebba V, Conte MP, Lepanto MS, Di Nardo G, Santangelo F, Aloi M, Totino V, Checchi MP, Longhi C, Cucchiara S, Schippa S. Iebba V, et al. Infect Immun. 2012 Apr;80(4):1408-17. doi: 10.1128/IAI.06181-11. Epub 2012 Jan 30. Infect Immun. 2012. PMID: 22290143 Free PMC article. - Adherent-invasive Escherichia coli in inflammatory bowel disease.
Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, Barnich N, Ng SC, Colombel JF. Palmela C, et al. Gut. 2018 Mar;67(3):574-587. doi: 10.1136/gutjnl-2017-314903. Epub 2017 Nov 15. Gut. 2018. PMID: 29141957 Review. - Escherichia coli-host macrophage interactions in the pathogenesis of inflammatory bowel disease.
Tawfik A, Flanagan PK, Campbell BJ. Tawfik A, et al. World J Gastroenterol. 2014 Jul 21;20(27):8751-63. doi: 10.3748/wjg.v20.i27.8751. World J Gastroenterol. 2014. PMID: 25083050 Free PMC article. Review.
Cited by
- Current Hypothesis for the Relationship between Dietary Rice Bran Intake, the Intestinal Microbiota and Colorectal Cancer Prevention.
So WK, Law BM, Law PT, Chan CW, Chair SY. So WK, et al. Nutrients. 2016 Sep 15;8(9):569. doi: 10.3390/nu8090569. Nutrients. 2016. PMID: 27649240 Free PMC article. Review. - Colibactin: More Than a New Bacterial Toxin.
Faïs T, Delmas J, Barnich N, Bonnet R, Dalmasso G. Faïs T, et al. Toxins (Basel). 2018 Apr 10;10(4):151. doi: 10.3390/toxins10040151. Toxins (Basel). 2018. PMID: 29642622 Free PMC article. Review. - Bacterial Genotoxin-Induced DNA Damage and Modulation of the Host Immune Microenvironment.
Martin OCB, Frisan T. Martin OCB, et al. Toxins (Basel). 2020 Jan 21;12(2):63. doi: 10.3390/toxins12020063. Toxins (Basel). 2020. PMID: 31973033 Free PMC article. Review. - Microbiota-derived small molecule genotoxins: host interactions and ecological impact in the gut ecosystem.
Zechner EL, Kienesberger S. Zechner EL, et al. Gut Microbes. 2024 Jan-Dec;16(1):2430423. doi: 10.1080/19490976.2024.2430423. Epub 2024 Nov 18. Gut Microbes. 2024. PMID: 39558480 Free PMC article. Review. - Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer.
Song M, Chan AT, Sun J. Song M, et al. Gastroenterology. 2020 Jan;158(2):322-340. doi: 10.1053/j.gastro.2019.06.048. Epub 2019 Oct 3. Gastroenterology. 2020. PMID: 31586566 Free PMC article. Review.
References
- Flanagan P, Campbell BJ, Rhodes JM. Bacteria in the pathogenesis of inflammatory bowel disease. Biochem Soc Trans 2011;39:1067–72 - PubMed
- Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 1998;115:1405–13 - PubMed
- Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, et al. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by PCR-denaturing gradient gel electrophoresis. Inflamm Bowel Dis 2006;12:1136–45 - PubMed
- Martinez-Medina M, Aldeguer X, Lopez-Siles M, et al. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm Bowel Dis 2009;15:872–82 - PubMed
- Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 2004;127:80–93 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous