Widespread evidence of cooperative DNA binding by transcription factors in Drosophila development - PubMed (original) (raw)
Widespread evidence of cooperative DNA binding by transcription factors in Drosophila development
Majid Kazemian et al. Nucleic Acids Res. 2013 Sep.
Abstract
Regulation of eukaryotic gene transcription is often combinatorial in nature, with multiple transcription factors (TFs) regulating common target genes, often through direct or indirect mutual interactions. Many individual examples of cooperative binding by directly interacting TFs have been identified, but it remains unclear how pervasive this mechanism is during animal development. Cooperative TF binding should be manifest in genomic sequences as biased arrangements of TF-binding sites. Here, we explore the extent and diversity of such arrangements related to gene regulation during Drosophila embryogenesis. We used the DNA-binding specificities of 322 TFs along with chromatin accessibility information to identify enriched spacing and orientation patterns of TF-binding site pairs. We developed a new statistical approach for this task, specifically designed to accurately assess inter-site spacing biases while accounting for the phenomenon of homotypic site clustering commonly observed in developmental regulatory regions. We observed a large number of short-range distance preferences between TF-binding site pairs, including examples where the preference depends on the relative orientation of the binding sites. To test whether these binding site patterns reflect physical interactions between the corresponding TFs, we analyzed 27 TF pairs whose binding sites exhibited short distance preferences. In vitro protein-protein binding experiments revealed that >65% of these TF pairs can directly interact with each other. For five pairs, we further demonstrate that they bind cooperatively to DNA if both sites are present with the preferred spacing. This study demonstrates how DNA-binding motifs can be used to produce a comprehensive map of sequence signatures for different mechanisms of combinatorial TF action.
Figures
Figure 1.
Schematic view of various site arrangements. (A) Types of site arrangement bias: orientation, distance and OSD bias. (B) Naming conventions (left panel) and instances (right panel) of relative orientations. The first two arrangements are equivalent for homotypic sites.
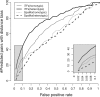
Figure 2.
Comparison between iTFs and SpaMo. For each method, the graph shows the number of predicted TF pairs (of 100) with distance bias as the significance level is varied. The _X_-axis shows the FPR corresponding to each significance level as estimated from randomized data. The thin and thick lines correspond to homotypic and heterotypic TF pairs, respectively.
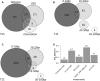
Figure 3.
Frequencies of different site arrangement biases. (A) Venn diagram of various site arrangement biases involving a total of 711 TF pairs. (B and C) For each of the four examined distance ranges, shown are the number of TF pairs with significant OSD bias (B) and distance bias (C) in that range. (D) Average number of partners with site arrangement biases, per TF, separated by TF families. (Only TF families with >10 TFs included in our analysis are shown.) The number of TFs in each family is shown in parentheses.
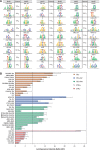
Figure 4.
Experimental validation of predicted TF–TF interactions. (A) Predicted site arrangement signatures. For each pair of motifs (first and third column), the second column shows the predicted distance bias. The ‘.rev’ extension next to the motif names indicates that the motif is in reverse complement orientation. (B) Measurement of direct in vitro interaction between TF pairs. TF pairs (listed on _Y_-axis) were expressed as fusions to MBP or luciferase (Luc). The recovery of Luc-tagged protein following an MBP pull-down is reported as the LIR with a threshold of LIR = 7 for positive interactions. Interactions are color coded to indicate those that were predicted to interact in the current study (iTFs), those acting in the anterior-posterior patterning network (AP), those have been previously reported in high-throughput PPI assays and positive or negative controls (CTRL+, CTRL−).
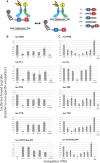
Figure 5.
Experimental validation of cooperative DNA binding for five selected TF pairs. (A) Schematic of assay to measure relative binding to pairs of DNA-binding sites. A biotinylated DNA probe with a wild-type (wt) DNA sequence containing a pair of binding sites for one (homotypic) or two (heterotypic) TFs is mixed with an excess of competitor DNA with either the wild-type or a variant DNA sequence. For homotypic interactions, a TF is labeled with luciferase (Luc). For heterotypic interactions, a second TF is labeled with MBP. The amount of Luc-tagged TF recovered with streptavidin beads reflects the relative affinity of the different competitor sequences for the tagged TF. Some of the DNA sequence variants tested include mutations that disrupt one (ΔA or ΔB) or both (ΔAB) of the TF-binding sites as well as insertions or deletions that change the spacing between the two sites. (B and C) DNA-binding site measurements for five homo or heterotypic TF–TF interactions. In each experiment, the biotinylated DNA probe is the wild-type (wt) sequence or not included (‘no probe’). The competitor DNA used is indicated on the _X_-axis. For ΔA + ΔB, the competitors with mutations in the individual TF-binding sites were used together, each at the concentration used for the individual competitor DNAs in the other samples. The recovered luciferase activity in the presence of the different competitors is shown on the _Y_-axis. The luciferase activity recovered using a competitor sequence with mutations in both TF-binding sites (ΔAB) was selected as representative of non-specific DNA competition; all other samples were reported as a fraction of the value of this sample. Changes in either the individual TF-binding sites or in the spacing between the binding sites result in reduced binding to the competitor DNA and an increased recovery of Luc-TF with the biotin-labeled DNA.
Similar articles
- A biophysical model for analysis of transcription factor interaction and binding site arrangement from genome-wide binding data.
He X, Chen CC, Hong F, Fang F, Sinha S, Ng HH, Zhong S. He X, et al. PLoS One. 2009 Dec 1;4(12):e8155. doi: 10.1371/journal.pone.0008155. PLoS One. 2009. PMID: 19956545 Free PMC article. - Computational identification of diverse mechanisms underlying transcription factor-DNA occupancy.
Cheng Q, Kazemian M, Pham H, Blatti C, Celniker SE, Wolfe SA, Brodsky MH, Sinha S. Cheng Q, et al. PLoS Genet. 2013;9(8):e1003571. doi: 10.1371/journal.pgen.1003571. Epub 2013 Aug 1. PLoS Genet. 2013. PMID: 23935523 Free PMC article. - CCAT: Combinatorial Code Analysis Tool for transcriptional regulation.
Jiang P, Singh M. Jiang P, et al. Nucleic Acids Res. 2014 Mar;42(5):2833-47. doi: 10.1093/nar/gkt1302. Epub 2013 Dec 23. Nucleic Acids Res. 2014. PMID: 24366875 Free PMC article. - Order through disorder: The role of intrinsically disordered regions in transcription factor binding specificity.
Brodsky S, Jana T, Barkai N. Brodsky S, et al. Curr Opin Struct Biol. 2021 Dec;71:110-115. doi: 10.1016/j.sbi.2021.06.011. Epub 2021 Jul 21. Curr Opin Struct Biol. 2021. PMID: 34303077 Review. - Sequence and chromatin determinants of transcription factor binding and the establishment of cell type-specific binding patterns.
Srivastava D, Mahony S. Srivastava D, et al. Biochim Biophys Acta Gene Regul Mech. 2020 Jun;1863(6):194443. doi: 10.1016/j.bbagrm.2019.194443. Epub 2019 Oct 19. Biochim Biophys Acta Gene Regul Mech. 2020. PMID: 31639474 Free PMC article. Review.
Cited by
- Asymmetric Conservation within Pairs of Co-Occurred Motifs Mediates Weak Direct Binding of Transcription Factors in ChIP-Seq Data.
Levitsky V, Oshchepkov D, Zemlyanskaya E, Merkulova T. Levitsky V, et al. Int J Mol Sci. 2020 Aug 21;21(17):6023. doi: 10.3390/ijms21176023. Int J Mol Sci. 2020. PMID: 32825662 Free PMC article. - Strand asymmetries across genomic processes.
Moeckel C, Zaravinos A, Georgakopoulos-Soares I. Moeckel C, et al. Comput Struct Biotechnol J. 2023 Mar 11;21:2036-2047. doi: 10.1016/j.csbj.2023.03.007. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 36968020 Free PMC article. Review. - Absence of a simple code: how transcription factors read the genome.
Slattery M, Zhou T, Yang L, Dantas Machado AC, Gordân R, Rohs R. Slattery M, et al. Trends Biochem Sci. 2014 Sep;39(9):381-99. doi: 10.1016/j.tibs.2014.07.002. Epub 2014 Aug 14. Trends Biochem Sci. 2014. PMID: 25129887 Free PMC article. Review. - Quantitative perturbation-based analysis of gene expression predicts enhancer activity in early Drosophila embryo.
Sayal R, Dresch JM, Pushel I, Taylor BR, Arnosti DN. Sayal R, et al. Elife. 2016 May 6;5:e08445. doi: 10.7554/eLife.08445. Elife. 2016. PMID: 27152947 Free PMC article. - ChIPulate: A comprehensive ChIP-seq simulation pipeline.
Datta V, Hannenhalli S, Siddharthan R. Datta V, et al. PLoS Comput Biol. 2019 Mar 21;15(3):e1006921. doi: 10.1371/journal.pcbi.1006921. eCollection 2019 Mar. PLoS Comput Biol. 2019. PMID: 30897079 Free PMC article.
References
- Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. Dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes Dev. 1992;6:1518–1530. - PubMed
- Magnani L, Eeckhoute J, Lupien M. Pioneer factors: directing transcriptional regulators within the chromatin environment. Trends Genet. 2011;27:465–474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous