Use of a standardized MxA protein measurement-based assay for validation of assays for the assessment of neutralizing antibodies against interferon-β - PubMed (original) (raw)
. 2013 Nov;33(11):660-71.
doi: 10.1089/jir.2012.0079. Epub 2013 Jul 13.
Meena Subramanyam, Susan Goelz, Jaya Goyal, Vijay Jethwa, Wendy Jones, James G Files, Daniel Kramer, Chris Bird, Paula Dilger, Michael Tovey, Christophe Lallemand, Robin Thorpe
Affiliations
- PMID: 23848523
- PMCID: PMC3814979
- DOI: 10.1089/jir.2012.0079
Use of a standardized MxA protein measurement-based assay for validation of assays for the assessment of neutralizing antibodies against interferon-β
Meenu Wadhwa et al. J Interferon Cytokine Res. 2013 Nov.
Abstract
Effective monitoring of the development of neutralizing antibodies (NAbs) against IFN-β in multiple sclerosis (MS) patients on IFN-β therapy is important for clinical decision making and disease management. To date, antiviral assays have been the favored approach for NAb determination, but variations in assay conditions between laboratories and the increasing use of novel assays have contributed to the reporting of inconsistent antibody data between laboratories and between products. This study, undertaken at the request of the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA), is a joint effort by manufacturers of IFN-β products (approved in Europe) towards harmonization of a NAb assay that facilitates generation of comparable NAb data, which, in conjunction with clinical outcomes, should prove useful for clinicians treating MS patients with IFN-β products. This article describes the standardized cellular myxovirus resistance protein A (MxA) protein measurement-based assay for detection of IFN-β NAbs and its use for the validation of assays used for the quantitative determination of such antibodies. Although titers varied between laboratories and the products used, utilization of IFN-β1a rather than IFN-β1b as the challenge antigen produced more consistent results in the NAb assay. Adoption of the standardized assay improves comparability between laboratories circumventing problems that arise when different, nonstandardized assays are employed for immunogenicity assessment. Based on the data, the EMA recommended for standardization purposes, the use of IFN-β1a in NAb assays, independent of the therapeutic product used for therapy and validation of new NAb procedures against the standardized assay described.
Figures
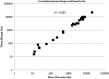
FIG. 1.
IFN-β NAb titers in antibody-positive patient sera using different antibody pairs in MxA ELISA for determination of MxA protein. IFN-β1a was used as the challenge antigen in the MxA assay; using Pearson's correlation, r=0.994 (_n_=28 for antibody-positive samples; _n_=31 in total). MxA, myxovirus resistance protein A; Nab, neutralizing antibody.
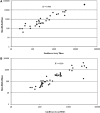
FIG. 2.
IFN-β NAb titers for antibody-positive patient sera using the MxA and luciferase NAb assays. (A) Using IFN-β1a as the challenge antigen (_n_=52 in total), the Pearson's correlation, r=0.951 for the antibody-positive samples (_n_=32). (B) Using IFN-β1b as the challenge antigen (_n_=65 in total), the Pearson's correlation, r=0.906 for the antibody-positive samples (_n_=45).
Similar articles
- Early detection of neutralizing antibodies to interferon-beta in multiple sclerosis patients: binding antibodies predict neutralizing antibody development.
Hegen H, Millonig A, Bertolotto A, Comabella M, Giovanonni G, Guger M, Hoelzl M, Khalil M, Killestein J, Lindberg R, Malucchi S, Mehling M, Montalban X, Polman CH, Rudzki D, Schautzer F, Sellebjerg F, Sørensen PS, Deisenhammer F. Hegen H, et al. Mult Scler. 2014 Apr;20(5):577-87. doi: 10.1177/1352458513503597. Epub 2013 Sep 5. Mult Scler. 2014. PMID: 24009164 - Incorporation of an interferon-β neutralizing antibody assay into routine clinical practice.
Farrell RA, Espasandin M, Lakdawala N, Creeke PI, Worthington V, Giovannoni G. Farrell RA, et al. Mult Scler. 2011 Nov;17(11):1333-40. doi: 10.1177/1352458511412654. Epub 2011 Jun 17. Mult Scler. 2011. PMID: 21685230 - Development and characterization of a non-cell-based assay to assess the presence of neutralizing antibodies to interferon-beta in clinical samples.
Cludts I, Meager A, Thorpe R, Wadhwa M. Cludts I, et al. J Immunol Methods. 2013 Sep 30;395(1-2):37-44. doi: 10.1016/j.jim.2013.06.008. Epub 2013 Jul 2. J Immunol Methods. 2013. PMID: 23831137 - Neutralizing antibodies to interferon.
Noronha A. Noronha A. Neurology. 2007 Jun 12;68(24 Suppl 4):S16-22. doi: 10.1212/01.wnl.0000277705.63813.84. Neurology. 2007. PMID: 17562846 Review. - Biological monitoring of IFN-β therapy in Multiple Sclerosis.
Bertolotto A, Granieri L, Marnetto F, Valentino P, Sala A, Capobianco M, Malucchi S, Di Sapio A, Malentacchi M, Matta M, Caldano M. Bertolotto A, et al. Cytokine Growth Factor Rev. 2015 Apr;26(2):241-8. doi: 10.1016/j.cytogfr.2014.12.002. Epub 2014 Dec 24. Cytokine Growth Factor Rev. 2015. PMID: 25596967 Review.
Cited by
- Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms.
Pellerin A, Otero K, Czerkowicz JM, Kerns HM, Shapiro RI, Ranger AM, Otipoby KL, Taylor FR, Cameron TO, Viney JL, Rabah D. Pellerin A, et al. EMBO Mol Med. 2015 Apr;7(4):464-76. doi: 10.15252/emmm.201404719. EMBO Mol Med. 2015. PMID: 25762615 Free PMC article. - Interferon score is increased in incomplete systemic lupus erythematosus and correlates with myxovirus-resistance protein A in blood and skin.
Lambers WM, de Leeuw K, Doornbos-van der Meer B, Diercks GFH, Bootsma H, Westra J. Lambers WM, et al. Arthritis Res Ther. 2019 Dec 2;21(1):260. doi: 10.1186/s13075-019-2034-4. Arthritis Res Ther. 2019. PMID: 31791398 Free PMC article. - Drug Efficacy Monitoring in Pharmacotherapy of Multiple Sclerosis With Biological Agents.
Caldano M, Raoul W, Rispens T, Bertolotto A. Caldano M, et al. Ther Drug Monit. 2017 Aug;39(4):350-355. doi: 10.1097/FTD.0000000000000393. Ther Drug Monit. 2017. PMID: 28328761 Free PMC article. Review.
References
- Aarskog N. Marøy T. Myhr K. Vedeler C. Antibodies against interferon-beta in multiple sclerosis. J Neuroimmunol. 2009;212:148–150. - PubMed
- Bertolotto A. Implications of neutralising antibodies on therapeutic efficacy. J Neurol Sci. 2009;277(Suppl 1):S29–S32. - PubMed
- Bertolotto A. Deisenhammer F. Gallo P. Sölberg Sørensen P. Immunogenicity of interferon beta: differences among products. J Neurol. 2004;251(Suppl 2):II15–II24. - PubMed
- Bertolotto A. Gilli F. Sala A. Capobianco M. Malucchi S. Milano E. Melis F. Marnetto F. Lindberg RL. Bottero R. Di Sapio A. Giordana MT. Persistent neutralizing antibodies abolish the interferon beta bioavailability in MS patients. Neurology. 2003;60:634–639. - PubMed
- Bertolotto A. Sala A. Caldano M. Capobianco M. Malucchi S. Development and validation of a real time PCR-based bioassay for quantification of neutralizing antibodies against human interferon-beta. J Immunol Methods. 2007;321:19–31. others. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials