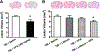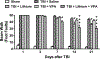Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury - PubMed (original) (raw)
Comparative Study
. 2013 Sep;119(3):766-73.
doi: 10.3171/2013.6.JNS13135. Epub 2013 Jul 12.
Affiliations
- PMID: 23848820
- PMCID: PMC6322557
- DOI: 10.3171/2013.6.JNS13135
Comparative Study
Posttrauma cotreatment with lithium and valproate: reduction of lesion volume, attenuation of blood-brain barrier disruption, and improvement in motor coordination in mice with traumatic brain injury
Fengshan Yu et al. J Neurosurg. 2013 Sep.
Abstract
Object: Although traumatic brain injury (TBI) is the leading cause of death and morbidity in young adults, no effective pharmaceutical treatment is available. By inhibiting glycogen synthase kinase-3 (GSK-3) and histone deacetylases (HDACs), respectively, lithium and valproate (VPA) have beneficial effects in diverse neurodegenerative diseases. Furthermore, in an excitotoxic neuronal model and in animal models of amyotrophic lateral sclerosis, Huntington disease, and stroke, combined treatment with lithium and VPA produces more robust neuroprotective effects than treatment with either agent alone. Building on previous work that establishes that therapeutic doses of either lithium or VPA have beneficial effects in mouse models of TBI, this study evaluated the effects of combined treatment with subeffective doses of lithium and VPA in a mouse model of TBI.
Methods: Male C57BL/6 mice underwent TBI and were subsequently treated with lithium, VPA, or a combination of lithium and VPA 15 minutes post-TBI and once daily thereafter for up to 3 weeks; all doses were subeffective (1 mEq/kg of lithium and 200 mg/kg of VPA). Assessed parameters included lesion volume via H & E staining; blood-brain barrier (BBB) integrity via immunoglobulin G extravasation; neurodegeneration via Fluoro-Jade B staining; motor coordination via a beam-walk test; and protein levels of acetylhistone H3, phospho-GSK-3β, and β-catenin via Western blotting.
Results: Posttrauma treatment with combined subeffective doses of lithium and VPA significantly reduced lesion volume, attenuated BBB disruption, and mitigated hippocampal neurodegeneration 3 days after TBI. As expected, subeffective doses of lithium or VPA alone did not have these beneficial effects. Combined treatment also improved motor coordination starting from Day 7 and persisting at least 21 days after TBI. Acetylation of histone H3, an index of HDAC inhibition, was robustly increased by the combined treatment 3 days after TBI.
Conclusions: Cotreatment with subeffective doses of lithium and VPA significantly attenuated TBI-induced brain lesion, BBB disruption, and neurodegeneration, and robustly improved long-term functional recovery. These findings suggest that potentiating histone acetylation by HDAC inhibition is probably part of the mechanism underlying the beneficial effects associated with this combined treatment for TBI. Because both lithium and VPA have a long history of safe clinical use, the results suggest that using a combination of these 2 agents at subtherapeutic doses to treat patients with TBI may also reduce side effects and enhance tolerability.
Figures
Fig. 1.
Combined treatment with subeffective doses of lithium and VPA reduced lesion volume 3 days post-TBI. H & E staining was performed to evaluate TBI-induced lesion volume. Representative H & E staining showed that TBI resulted in lesions in the ipsilateral cortex and hippocampus. A: Treatment with 300 mg/kg VPA significantly reduced lesion volume 3 days after TBI (n = 8/group; *p < 0.05). B: Treatment with lithium at 1 mEq/kg or VPA at 200 mg/kg had no effect on lesion volume. Combined treatment with these subeffective doses of lithium and VPA significantly reduced lesion volume (B; n = 9/group; *p < 0.05). Data throughout are expressed as the mean ± SEM.
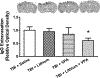
Fig. 2.
Combined treatment with subeffective doses of lithium and VPA attenuated BBB disruption 3 days after TBI. IgG staining was used to determine BBB disruption. Representative photographs (upper row) showing TBI increased the immune-positive signal in the area close to injury. The immune reactivity in monotreatment groups with either lithium or VPA was similar to that in the saline-treated group, whereas combined treatment significantly reduced the area of IgG-positive staining. Quantitative analysis showed that the relative optical density of IgG staining was robustly reduced after combined treatment, compared with the saline-treated group (n = 8/group; *p < 0.05), whereas treatment with lithium or VPA alone did not affect relative optical density.
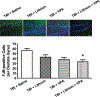
Fig. 3.
Combined treatment with subeffective doses of lithium and VPA reduced the number of degenerating neurons 3 days post-TBI. Neuronal degeneration was evaluated using FJB staining. Representative microphotographs (upper row) showing FJB staining in the hippocampal dentate gyrus 3 days after injury. In the saline-treated group, many FJB-positive cells were visualized. Although fewer FJB-positive cells were observed in response to lithium or VPA treatment alone, the combined treatment group showed the fewest FJB-positive cells. Compared with the saline-treated group, quantitative analysis showed that the number of FJB-positive cells was significantly reduced in the combined treatment group (n = 9/group; *p < 0.05). Nuclei were stained with 4,6-diamino-2-phenylindole, as shown with blue fluorescence. Bar = 50 μm.
Fig. 4.
Combined treatment with subeffective doses of lithium and VPA improved beam-walk performance after TBI. The beam-walk test was performed 1, 3, 7, 14, and 21 days after injury to assess motor coordination. TBI markedly increased the number of foot faults in all groups examined. A slow recovery from 7 to 21 days postinjury was seen in all groups. Compared with the saline-treated group, a significant reduction in the number of foot faults was observed in the combined treatment group 7, 14, and 21 days after injury (n = 8/group for all drug treatment groups; n = 6/group for the sham and saline groups; *p < 0.05; ***p < 0.001). Treatment with VPA alone did not affect the number of foot faults at any time point, whereas the lithium-treated group showed a reduced number of foot faults compared with the saline-treated group on Days 14 and 21 after injury.
Fig. 5.
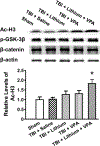
Combined treatment with subeffective doses of lithium and VPA increased levels of Ac-H3 3 days after TBI. The Ac-H3 levels remained unchanged in the ipsilateral cortex in the saline-treated group compared with the sham-operated group. After treatment with lithium or VPA alone, levels of Ac-H3 increased slightly. Combined treatment significantly increased Ac-H3 levels compared with the saline-treated group (upper). Quantified data showed a significant increase in Ac-H3 levels after combined treatment compared with the saline-treated group (lower; n = 6/group; *p < 0.05). Levels of phospho-GSK-3β (p-GSK-3β) and β-catenin were unchanged after injury or any treatment. The β-actin level was measured as a loading control.
Similar articles
- Combined treatment with the mood stabilizers lithium and valproate produces multiple beneficial effects in transgenic mouse models of Huntington's disease.
Chiu CT, Liu G, Leeds P, Chuang DM. Chiu CT, et al. Neuropsychopharmacology. 2011 Nov;36(12):2406-21. doi: 10.1038/npp.2011.128. Epub 2011 Jul 27. Neuropsychopharmacology. 2011. PMID: 21796107 Free PMC article. - Lithium ameliorates neurodegeneration, suppresses neuroinflammation, and improves behavioral performance in a mouse model of traumatic brain injury.
Yu F, Wang Z, Tchantchou F, Chiu CT, Zhang Y, Chuang DM. Yu F, et al. J Neurotrauma. 2012 Jan 20;29(2):362-74. doi: 10.1089/neu.2011.1942. Epub 2011 Nov 1. J Neurotrauma. 2012. PMID: 21895523 Free PMC article. - Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats.
Dash PK, Orsi SA, Zhang M, Grill RJ, Pati S, Zhao J, Moore AN. Dash PK, et al. PLoS One. 2010 Jun 30;5(6):e11383. doi: 10.1371/journal.pone.0011383. PLoS One. 2010. PMID: 20614021 Free PMC article. - A new avenue for lithium: intervention in traumatic brain injury.
Leeds PR, Yu F, Wang Z, Chiu CT, Zhang Y, Leng Y, Linares GR, Chuang DM. Leeds PR, et al. ACS Chem Neurosci. 2014 Jun 18;5(6):422-33. doi: 10.1021/cn500040g. Epub 2014 Apr 11. ACS Chem Neurosci. 2014. PMID: 24697257 Free PMC article. Review. - The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials.
Chuang DM. Chuang DM. Ann N Y Acad Sci. 2005 Aug;1053:195-204. doi: 10.1196/annals.1344.018. Ann N Y Acad Sci. 2005. PMID: 16179524 Review.
Cited by
- Novel Mouse Tauopathy Model for Repetitive Mild Traumatic Brain Injury: Evaluation of Long-Term Effects on Cognition and Biomarker Levels After Therapeutic Inhibition of Tau Phosphorylation.
Rubenstein R, Sharma DR, Chang B, Oumata N, Cam M, Vaucelle L, Lindberg MF, Chiu A, Wisniewski T, Wang KKW, Meijer L. Rubenstein R, et al. Front Neurol. 2019 Mar 11;10:124. doi: 10.3389/fneur.2019.00124. eCollection 2019. Front Neurol. 2019. PMID: 30915013 Free PMC article. - Effects of lithium on inflammation.
Nassar A, Azab AN. Nassar A, et al. ACS Chem Neurosci. 2014 Jun 18;5(6):451-8. doi: 10.1021/cn500038f. Epub 2014 May 6. ACS Chem Neurosci. 2014. PMID: 24803181 Free PMC article. Review. - The role of the stress system in recovery after traumatic brain injury: A tribute to Bruce S. McEwen.
Weil ZM, White B, Whitehead B, Karelina K. Weil ZM, et al. Neurobiol Stress. 2022 Jun 4;19:100467. doi: 10.1016/j.ynstr.2022.100467. eCollection 2022 Jul. Neurobiol Stress. 2022. PMID: 35720260 Free PMC article. - Potential immunotherapies for traumatic brain and spinal cord injury.
Putatunda R, Bethea JR, Hu WH. Putatunda R, et al. Chin J Traumatol. 2018 Jun;21(3):125-136. doi: 10.1016/j.cjtee.2018.02.002. Epub 2018 Apr 18. Chin J Traumatol. 2018. PMID: 29759918 Free PMC article. Review. - The effect of mild traumatic brain injury on peripheral nervous system pathology in wild-type mice and the G93A mutant mouse model of motor neuron disease.
Evans TM, Jaramillo CA, Sataranatarajan K, Watts L, Sabia M, Qi W, Van Remmen H. Evans TM, et al. Neuroscience. 2015 Jul 9;298:410-23. doi: 10.1016/j.neuroscience.2015.04.041. Epub 2015 Apr 25. Neuroscience. 2015. PMID: 25921732 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials