The role of salt bridges, charge density, and subunit flexibility in determining disassembly routes of protein complexes - PubMed (original) (raw)
The role of salt bridges, charge density, and subunit flexibility in determining disassembly routes of protein complexes
Zoe Hall et al. Structure. 2013.
Abstract
Mass spectrometry can be used to characterize multiprotein complexes, defining their subunit stoichiometry and composition following solution disruption and collision-induced dissociation (CID). While CID of protein complexes in the gas phase typically results in the dissociation of unfolded subunits, a second atypical route is possible wherein compact subunits or subcomplexes are ejected without unfolding. Because tertiary structure and subunit interactions may be retained, this is the preferred route for structural investigations. How can we influence which pathway is adopted? By studying properties of a series of homomeric and heteromeric protein complexes and varying their overall charge in solution, we found that low subunit flexibility, higher charge densities, fewer salt bridges, and smaller interfaces are likely to be involved in promoting dissociation routes without unfolding. Manipulating the charge on a protein complex therefore enables us to direct dissociation through structurally informative pathways that mimic those followed in solution.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
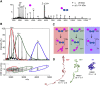
Graphical abstract
Figure 1
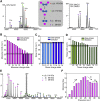
CID of Serum Amyloid P 5-mer (A) MS/MS of SAP 5-mer with increasing precursor ion charge state. MS/MS spectra were acquired at the same laboratory frame energy (collision energy × Z) with the exception of the lowest charge state (18+), where higher energies were required in order to effect dissociation. (B) Average charge state (Z_av_) of the dissociated monomer was plotted against the precursor ion charge state. For Z ≥ 25+, two populations of monomer are observed. (C) The relative intensities of monomer and dimer CID products in the MS/MS spectra are plotted against precursor ion charge state. Precursor charge states were generated as follows: to 200 mM AA: 18–21+ (10 mM TEA), 22–25+ (no additive), and 26–30+ (1% _m_-NBA). See also Figure S1.
Figure 2
CID of Transthyretin (A) MS/MS of TTR with increasing precursor ion charge state. Initially, the dissociated monomer charge state increases with the precursor ion charge state. Supercharged TTR (21+) can dissociate via alternate pathways, losing lower charged monomers and dimers. (B) Z_av_ of the dissociated monomer is plotted against precursor ion charge state. At Z ≥ 19+, two distinct populations of monomer are observed. (C) Relative intensities of monomer and dimer CID products are plotted against precursor ion charge state. Precursor charge states were generated as follows: to 200 mM AA: 10–13+ (20 mM DBU), 14–16+ (no additive), and 17–21+ (1% _m_-NBA). See also Figure S2.
Figure 3
Comparison of Gas Phase Dissociation and Solution Disassembly (A–H) SAP (orange/red) and TTR (cyan/blue) were disrupted in solution by the addition of acetonitrile (A) and (D). CCS were measured for the monomer formed in solution and compared with CCS of monomer resulting from gas phase dissociation (B and E), and values were calculated from the X-ray crystal structure (dashed line with ± 3% error shaded). CCS were also measured for dimer formed in solution, gas phase, and calculated from crystal structure (C and F). Dimer was not formed in solution for SAP. CCSs for each charge state (gas phase products) are the average value calculated from multiple MS/MS experiments of different precursor ions that give the same charge product ions (SD < 1%–2%). Multiple conformations are indicated by a or b for SAP monomer (8+) and dimer (11+). Schematics show solution disruption (G) and CID, with increasing precursor ion Z (H). See also Figure S3.
Figure 4
Gas Phase Dissociation of Tryptophan Synthase (A) MS/MS of tryptophan synthase (24+) reveals loss of α subunit. (B) Three distinct populations of ejected α subunit are observed and confirmed via a drift time versus m/z contour plot. (C) Schematic for the proposed parallel routes for gas phase dissociation: loss of high, intermediate, and low-charge subunits (red, green, and blue). (D) MD simulations of tryptophan synthase (24+), over a linear temperature gradient, recapitulates the ejection of an α-monomer with different degrees of compactness. Charges assigned to the α subunit were 14+, 11+, and 7+, as determined experimentally. Residual charges were evenly distributed over the accessible basic residues on the remaining three subunits. The rmsd of the dissociated α subunit from that bound in the native structure is shown. See also Figure S4.
Figure 5
Comparison of Tryptophan Synthase Gas and Solution Phase Disassembly (A) Tryptophan synthase dissembles in solution by the loss of α-monomers to give αβ2-trimer, β2-dimer and α−monomer, depending on solution conditions. (B–D) CCS were measured for the α-monomer (B), β2-dimer (C), and αβ2-trimer (D) formed in solution (purple, blue, pale green, respectively), and compared with CCS of the corresponding gas phase product (pink, cyan, green). Two conformations of the gas phase trimer 14+ are identified (a and b). (E) At higher collision energy (28+) two monomers dissociate, allowing CCS measurements of the β2-dimer. (F) Z_av_ for the dissociated α-monomer is plotted against precursor ion charge state. Charge states were generated as follows: to 200 mM AA: 19–21+ (20 mM TEA), 22–26+ (no additive), and 27–31+ (1% _m_-NBA). See also Figure S5.
Figure 6
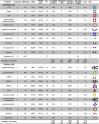
Properties of a Range of Multiprotein Complexes and Their Dissociation Pathways Complexes that dissociate atypically and typically are shown in the upper and lower parts of the table, respectively. aSASA was calculated using PISA. bZ_av_ is the average charge state observed in 1% _m_-NBA (this study) or reported under varying solution conditions. cMinimal number of interfacial salt bridges broken during the first step of in silico disassembly based on breaking the minimum interfacial surface area. Salt bridges were calculated using PISA (Krissinel and Henrick, 2007). dInterface area broken during first step of in silico disassembly. e_A_rel is the subunit relative SASA, and a proxy for flexibility. References: 1 (van den Heuvel et al., 2006); 2 (Boeri Erba et al., 2010); 3 (Zhou et al., 2013); 4 (Kükrer et al., 2012); 5 (Dodds et al., 2011); 6 (Loo, 2001); 7 (Versluis et al., 2001); 8 (Aquilina, 2009); and 9 (Fitzgerald et al., 1996). See also Figure S6 and Table S1.
Figure 7
In Silico Disassembly of Complexes Using the Interface Model In silico disassembly proceeds by the route in which the least amount of interface area is broken. The crystal structures of the complexes undergoing typical (A) or atypical CID (B) are shown with their corresponding PDB numbers, and ball-and-stick representations (ball, monomer; stick, interface). Interface areas in Å2 (black) and number of salt bridges (red) are marked. Interfacial salt bridges broken in each in silico disassembly step are given above the reaction arrows. Gas phase disassembly routes are defined as typical dissociation of an unfolded monomer (T) or fragment (T∗). Atypical dissociation is represented as follows: formation of subcomplexes A(1), loss of compact monomers A(2), and symmetric charge partitioning between dissociation products A(3). See also Figure S7.
Comment in
- Protein complexes: breaking up is hard to do well.
Loo RR, Loo JA. Loo RR, et al. Structure. 2013 Aug 6;21(8):1265-6. doi: 10.1016/j.str.2013.07.013. Structure. 2013. PMID: 23931137 Free PMC article.
Similar articles
- Protein complexes: breaking up is hard to do well.
Loo RR, Loo JA. Loo RR, et al. Structure. 2013 Aug 6;21(8):1265-6. doi: 10.1016/j.str.2013.07.013. Structure. 2013. PMID: 23931137 Free PMC article. - Charge-state dependent compaction and dissociation of protein complexes: insights from ion mobility and molecular dynamics.
Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV. Hall Z, et al. J Am Chem Soc. 2012 Feb 22;134(7):3429-38. doi: 10.1021/ja2096859. Epub 2012 Feb 13. J Am Chem Soc. 2012. PMID: 22280183 - Ion mobility-mass spectrometry reveals the influence of subunit packing and charge on the dissociation of multiprotein complexes.
Boeri Erba E, Ruotolo BT, Barsky D, Robinson CV. Boeri Erba E, et al. Anal Chem. 2010 Dec 1;82(23):9702-10. doi: 10.1021/ac101778e. Epub 2010 Nov 5. Anal Chem. 2010. PMID: 21053918 - Structure, dynamics, assembly, and evolution of protein complexes.
Marsh JA, Teichmann SA. Marsh JA, et al. Annu Rev Biochem. 2015;84:551-75. doi: 10.1146/annurev-biochem-060614-034142. Epub 2014 Dec 8. Annu Rev Biochem. 2015. PMID: 25494300 Review. - Structural Reconstruction of Protein-Protein Complexes Involved in Intracellular Signaling.
Kirsch K, Sok P, Reményi A. Kirsch K, et al. Adv Exp Med Biol. 2016;896:315-26. doi: 10.1007/978-3-319-27216-0_20. Adv Exp Med Biol. 2016. PMID: 27165334 Review.
Cited by
- Elucidating Structures of Protein Complexes by Collision-Induced Dissociation at Elevated Gas Pressures.
Liu FC, Cropley TC, Bleiholder C. Liu FC, et al. J Am Soc Mass Spectrom. 2023 Oct 4;34(10):2247-2258. doi: 10.1021/jasms.3c00191. Epub 2023 Sep 20. J Am Soc Mass Spectrom. 2023. PMID: 37729591 Free PMC article. - Simulation toolkits at the molecular scale for trans-scale thermal signaling.
Kurisaki I, Suzuki M. Kurisaki I, et al. Comput Struct Biotechnol J. 2023 Mar 24;21:2547-2557. doi: 10.1016/j.csbj.2023.03.040. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37102156 Free PMC article. Review. - Transthyretin proteoforms of intraocular origin in human subretinal fluid.
Chen J, Cao D, Fortmann SD, Curcio CA, Feist RM, Crosson JN. Chen J, et al. Exp Eye Res. 2022 Sep;222:109163. doi: 10.1016/j.exer.2022.109163. Epub 2022 Jun 26. Exp Eye Res. 2022. PMID: 35760119 Free PMC article. - Rapid structural discrimination of IgG antibodies by multicharge-state collision-induced unfolding.
Yin Z, Du M, Chen D, Zhang W, Huang W, Wu X, Yan S. Yin Z, et al. RSC Adv. 2021 Nov 12;11(58):36502-36510. doi: 10.1039/d1ra06486j. eCollection 2021 Nov 10. RSC Adv. 2021. PMID: 35494361 Free PMC article. - Surface-induced Dissociation Mass Spectrometry as a Structural Biology Tool.
Snyder DT, Harvey SR, Wysocki VH. Snyder DT, et al. Chem Rev. 2022 Apr 27;122(8):7442-7487. doi: 10.1021/acs.chemrev.1c00309. Epub 2021 Nov 2. Chem Rev. 2022. PMID: 34726898 Free PMC article. Review.
References
- Aquilina J.A. The major toxin from the Australian common brown snake is a hexamer with unusual gas-phase dissociation properties. Proteins. 2009;75:478–485. - PubMed
- Benesch J.L., Aquilina J.A., Ruotolo B.T., Sobott F., Robinson C.V. Tandem mass spectrometry reveals the quaternary organization of macromolecular assemblies. Chem. Biol. 2006;13:597–605. - PubMed
- Boeri Erba E., Ruotolo B.T., Barsky D., Robinson C.V. Ion mobility-mass spectrometry reveals the influence of subunit packing and charge on the dissociation of multiprotein complexes. Anal. Chem. 2010;82:9702–9710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases