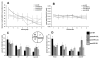Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer's disease - PubMed (original) (raw)
Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer's disease
Sarah M Rothman et al. Brain Res. 2013.
Abstract
Age-associated dysregulation of sleep can be worsened by Alzheimer's disease (AD). AD and sleep restriction both impair cognition, yet it is unknown if mild chronic sleep restriction modifies the proteopathic processes involved in AD. The goal of this work was to test the hypothesis that sleep restriction worsens memory impairments, and amyloid β-peptide (Aβ) and pTau accumulations in the brain in a mouse model of AD, with a focus on a role for circulating glucocorticoids (GC). Male 3xTgAD mice were subjected to sleep restriction (SR) for 6h/day for 6 weeks using the modified multiple platform technique, and behavioral (Morris water maze, fear conditioning, open field) and biochemical (immunoblot) outcomes were compared to mice undergoing daily cage transfers (large cage control; LCC) as well as control mice that remained in their home cage (control; CTL). At one week, both LCC and SR mice displayed significant elevations in plasma corticosterone compared to CTL (p<0.002). By four weeks, SR mice displayed a two-fold increase in circulating corticosterone levels compared to CTL. Behavioral data indicated deficits in contextual and cued memory in SR mice that were not present for LCC or CTL (p<0.04). Both Aβ and pTau levels increased in the cortex of SR mice compared to CTL and LCC; however these changes were not noted in the hippocampus. Significant positive correlations between cortical Aβ and pTau levels and circulating corticosterone indicate a potential role for GCs in mediating behavioral and biochemical changes observed after sleep restriction in a mouse model of AD.
Keywords: AD; Alzheimer′s disease; Amyloid; Aβ; CTL; Fear conditioning; GC; Glucocorticoids; LCC; MMP; MWM; Morris water maze; SR; Sleep restriction; amyloid beta; control; glucocorticoid; large cage control; modified multiple platform; pTau; phosphorylated tau; sleep restriction.
Published by Elsevier B.V.
Conflict of interest statement
Conflict of Interest
The authors report no conflicts of interest.
Figures
Figure 1
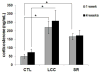
Blood plasma corticosterone in control (CTL), large cage control (LCC) and sleep restriction (SR) mice at 1 and 4 weeks after the start of the 6-week sleep restriction period.
Figure 2
Morris water maze results for all mice prior to sleep restriction (pre-SR) and after the 6 week sleep restriction period for control (post-SR CTL), large cage control (post-SR LCC) and sleep restricted mice (post-SR SR). The latency time to reach the hidden platform decreased over time for mice tested prior to the sleep restriction period (A). Mean swim speed was not significantly different between groups (B). At both the 4 and 24 hour probe test (C&D), mice tested prior to the sleep restriction period spent a significantly greater amount of time in the target quadrant (p<0.005) whereas after the 6 week sleep restriction period no groups, including control, displayed significant preference for the target quadrant, indicating a lack of short term memory retention.
Figure 3
Fear conditioning testing results for all mice after the 6 week sleep restriction period for control (post-SR CTL), large cage control (post-SR LCC) and sleep restricted mice (post-SR SR). On day 1, mice were exposed to tone-shock pairings (A). On day 2, mice were first tested in a contextual paradigm (B) followed by a cued paradigm (C).
Figure 4
After the 6 week sleep restriction period, no differences in the time spent in zone 1 (A) or the distance traveled in zone 1 (B) are observed between groups, implying that there are no differences in anxiety between the three groups (CTL, LCC, SR).
Figure 5
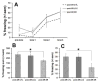
Western blot results from the cortex (A) and hippocampus (B) showing increases in expression of Aβ and ptau in large cage controls compared to control with a further increase in sleep restricted mice. Quantification of western blot results indicate that cortical Aβ expression increases in LCC and SR compared to CTL whereas ptau expression increases in SR (C). Hippocampal expression levels are largely unchanged between groups (D).
Figure 6
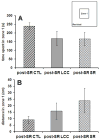
Correlations between circulating glucocorticoids and hippocampal and cortical expression of Aβ and ptau. Cortical expression of both Aβ and ptau are positively, significantly (p<0.028) correlated with circulating glucocorticoids measured 1 week after the start of the sleep restriction period (A–B). In the hippocampus, Aβ, but not ptau, is significantly correlated with circulating glucocorticoids (p=0.032; C–D). Circulating corticosterone was negatively and significantly (p=0.045) correlated with the percent time spent in the target quadrant during the 24 hour probe test (E).
Similar articles
- 3xTgAD mice exhibit altered behavior and elevated Aβ after chronic mild social stress.
Rothman SM, Herdener N, Camandola S, Texel SJ, Mughal MR, Cong WN, Martin B, Mattson MP. Rothman SM, et al. Neurobiol Aging. 2012 Apr;33(4):830.e1-12. doi: 10.1016/j.neurobiolaging.2011.07.005. Epub 2011 Aug 19. Neurobiol Aging. 2012. PMID: 21855175 Free PMC article. - Voluntary Running Attenuates Memory Loss, Decreases Neuropathological Changes and Induces Neurogenesis in a Mouse Model of Alzheimer's Disease.
Tapia-Rojas C, Aranguiz F, Varela-Nallar L, Inestrosa NC. Tapia-Rojas C, et al. Brain Pathol. 2016 Jan;26(1):62-74. doi: 10.1111/bpa.12255. Epub 2015 May 7. Brain Pathol. 2016. PMID: 25763997 Free PMC article. - Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice.
España J, Giménez-Llort L, Valero J, Miñano A, Rábano A, Rodriguez-Alvarez J, LaFerla FM, Saura CA. España J, et al. Biol Psychiatry. 2010 Mar 15;67(6):513-21. doi: 10.1016/j.biopsych.2009.06.015. Epub 2009 Aug 7. Biol Psychiatry. 2010. PMID: 19664757 - Cognitive Decline in Preclinical Alzheimer's Disease: Amyloid-Beta versus Tauopathy.
Huber CM, Yee C, May T, Dhanala A, Mitchell CS. Huber CM, et al. J Alzheimers Dis. 2018;61(1):265-281. doi: 10.3233/JAD-170490. J Alzheimers Dis. 2018. PMID: 29154274 Free PMC article. Review. - Alcohol drinking exacerbates neural and behavioral pathology in the 3xTg-AD mouse model of Alzheimer's disease.
Hoffman JL, Faccidomo S, Kim M, Taylor SM, Agoglia AE, May AM, Smith EN, Wong LC, Hodge CW. Hoffman JL, et al. Int Rev Neurobiol. 2019;148:169-230. doi: 10.1016/bs.irn.2019.10.017. Epub 2019 Oct 23. Int Rev Neurobiol. 2019. PMID: 31733664 Free PMC article. Review.
Cited by
- Pathobiolgy and Management of Alzheimer's Disease.
Kim H, Chung JY. Kim H, et al. Chonnam Med J. 2021 May;57(2):108-117. doi: 10.4068/cmj.2021.57.2.108. Epub 2021 May 24. Chonnam Med J. 2021. PMID: 34123738 Free PMC article. Review. - Life Experience Matters: Enrichment and Stress Can Influence the Likelihood of Developing Alzheimer's Disease via Gut Microbiome.
Torraville SE, Flynn CM, Kendall TL, Yuan Q. Torraville SE, et al. Biomedicines. 2023 Jul 3;11(7):1884. doi: 10.3390/biomedicines11071884. Biomedicines. 2023. PMID: 37509523 Free PMC article. Review. - Early Life Sleep Deprivation and Brain Development: Insights From Human and Animal Studies.
Alrousan G, Hassan A, Pillai AA, Atrooz F, Salim S. Alrousan G, et al. Front Neurosci. 2022 May 3;16:833786. doi: 10.3389/fnins.2022.833786. eCollection 2022. Front Neurosci. 2022. PMID: 35592259 Free PMC article. - The effect of insomnia on development of Alzheimer's disease.
Sadeghmousavi S, Eskian M, Rahmani F, Rezaei N. Sadeghmousavi S, et al. J Neuroinflammation. 2020 Oct 6;17(1):289. doi: 10.1186/s12974-020-01960-9. J Neuroinflammation. 2020. PMID: 33023629 Free PMC article. Review. - The Neurobiological Basis of Sleep and Sleep Disorders.
Joiner WJ. Joiner WJ. Physiology (Bethesda). 2018 Sep 1;33(5):317-327. doi: 10.1152/physiol.00013.2018. Physiology (Bethesda). 2018. PMID: 30109824 Free PMC article. Review.
References
- Bliwise DL, Tinklenberg J, Yesavage JA, Davies H, Pursley AM, Petta DE, Widrow L, Guilleminault C, Zarcone VP, Dement WC. REM latency in Alzheimer’s disease. Biol Psychiat. 1989;25:320–328. - PubMed
- Budas G, Coughlan CM, Seckl JR, Breen KC. The effect of corticosteroids on amyloid beta precursor protein/amyloid precursor-like protein expression and processing in vivo. Neurosci Lett. 1999;276:61–64. - PubMed
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiat. 2009;14:95–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- ZIA AG000317/ImNIH/Intramural NIH HHS/United States
- ZIA AG000330-04/ImNIH/Intramural NIH HHS/United States
- ZIA AG000317-11/ImNIH/Intramural NIH HHS/United States
- ZIA AG000312/ImNIH/Intramural NIH HHS/United States
- ZIA AG000317-12/ImNIH/Intramural NIH HHS/United States
- ZIA AG000312-12/ImNIH/Intramural NIH HHS/United States
- ZIA AG000312-11/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous