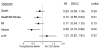Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial - PubMed (original) (raw)
Randomized Controlled Trial
Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial
Richard G Bach et al. Circulation. 2013.
Abstract
Background: Rosiglitazone improves glycemic control for patients with type 2 diabetes mellitus, but there remains controversy regarding an observed association with cardiovascular hazard. The cardiovascular effects of rosiglitazone for patients with coronary artery disease remain unknown.
Methods and results: To examine any association between rosiglitazone use and cardiovascular events among patients with diabetes mellitus and coronary artery disease, we analyzed events among 2368 patients with type 2 diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Total mortality, composite death, myocardial infarction, and stroke, and the individual incidence of death, myocardial infarction, stroke, congestive heart failure, and fractures, were compared during 4.5 years of follow-up among patients treated with rosiglitazone versus patients not receiving a thiazolidinedione by use of Cox proportional hazards and Kaplan-Meier analyses that included propensity matching. After multivariable adjustment, among patients treated with rosiglitazone, mortality was similar (hazard ratio [HR], 0.83; 95% confidence interval [CI], 0.58-1.18), whereas there was a lower incidence of composite death, myocardial infarction, and stroke (HR, 0.72; 95% CI, 0.55-0.93) and stroke (HR, 0.36; 95% CI, 0.16-0.86) and a higher incidence of fractures (HR, 1.62; 95% CI, 1.05-2.51); the incidence of myocardial infarction (HR, 0.77; 95% CI, 0.54-1.10) and congestive heart failure (HR, 1.22; 95% CI, 0.84-1.82) did not differ significantly. Among propensity-matched patients, rates of major ischemic cardiovascular events and congestive heart failure were not significantly different.
Conclusions: Among patients with type 2 diabetes mellitus and coronary artery disease in the BARI 2D trial, neither on-treatment nor propensity-matched analysis supported an association of rosiglitazone treatment with an increase in major ischemic cardiovascular events.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00006305.
Keywords: coronary disease; diabetes mellitus; myocardial infarction; pharmaceutical preparations; rosiglitazone; thiazolidinediones.
Figures
Figure 1
Relative risk of adverse cardiovascular outcomes for patients on treatment with rosiglitazone compared with patients not treated with a thiazolidinedione, after adjustment for baseline characteristics and other diabetes-related medications.
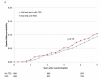
Figure 2
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Figure 2
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Figure 2
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Figure 2
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Figure 2
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Figure 2
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Comment in
- Rosiglitazone and cardiovascular outcomes: is there a clear answer?
Gerstein HC. Gerstein HC. Circulation. 2013 Aug 20;128(8):777-9. doi: 10.1161/CIRCULATIONAHA.113.004652. Epub 2013 Jul 15. Circulation. 2013. PMID: 23857319 No abstract available.
Similar articles
- Response to letter regarding article, "rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial".
Bach RG, Brooks MM, Lombardero M, Genuth S, Donner TW, Garber A, Kennedy L, Monrad ES, Pop-Busui R, Kelsey SF, Frye RL; BARI 2D Investigators. Bach RG, et al. Circulation. 2014 Apr 22;129(16):e460-1. doi: 10.1161/CIRCULATIONAHA.113.008033. Circulation. 2014. PMID: 24753557 No abstract available. - Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone.
Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Graham DJ, et al. JAMA. 2010 Jul 28;304(4):411-8. doi: 10.1001/jama.2010.920. Epub 2010 Jun 28. JAMA. 2010. PMID: 20584880 - Coronary revascularization in patients with type 2 diabetes and results of the BARI 2D trial.
Sobel BE. Sobel BE. Coron Artery Dis. 2010 May;21(3):189-98. doi: 10.1097/MCA.0b013e3283383ebe. Coron Artery Dis. 2010. PMID: 20308880 Review. - The safety of rosiglitazone in the treatment of type 2 diabetes.
Singh S, Loke YK. Singh S, et al. Expert Opin Drug Saf. 2008 Sep;7(5):579-85. doi: 10.1517/14740338.7.5.579. Expert Opin Drug Saf. 2008. PMID: 18759710 Review.
Cited by
- Rosiglitazone treatment and cardiovascular disease in the Veterans Affairs Diabetes Trial.
Florez H, Reaven PD, Bahn G, Moritz T, Warren S, Marks J, Reda D, Duckworth W, Abraira C, Hayward R, Emanuele N; VADT Research Group. Florez H, et al. Diabetes Obes Metab. 2015 Oct;17(10):949-55. doi: 10.1111/dom.12487. Epub 2015 Jun 17. Diabetes Obes Metab. 2015. PMID: 25964070 Free PMC article. Clinical Trial. - The forgotten type 2 diabetes mellitus medicine: rosiglitazone.
Xu B, Xing A, Li S. Xu B, et al. Diabetol Int. 2021 Jun 29;13(1):49-65. doi: 10.1007/s13340-021-00519-0. eCollection 2022 Jan. Diabetol Int. 2021. PMID: 35059243 Free PMC article. Review. - Insulin and glucose-lowering agents for treating people with diabetes and chronic kidney disease.
Lo C, Toyama T, Wang Y, Lin J, Hirakawa Y, Jun M, Cass A, Hawley CM, Pilmore H, Badve SV, Perkovic V, Zoungas S. Lo C, et al. Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD011798. doi: 10.1002/14651858.CD011798.pub2. Cochrane Database Syst Rev. 2018. PMID: 30246878 Free PMC article. - Pioglitazone and cardiovascular risk reduction: time for a second look?
Perdigoto AL, Young LH, Inzucchi SE. Perdigoto AL, et al. Cardiovasc Endocrinol. 2017 May 17;6(2):55-61. doi: 10.1097/XCE.0000000000000110. eCollection 2017 Jun. Cardiovasc Endocrinol. 2017. PMID: 31646121 Free PMC article. Review. - Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes.
Soccio RE, Chen ER, Lazar MA. Soccio RE, et al. Cell Metab. 2014 Oct 7;20(4):573-91. doi: 10.1016/j.cmet.2014.08.005. Epub 2014 Sep 18. Cell Metab. 2014. PMID: 25242225 Free PMC article. Review.
References
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. - PubMed
- Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM, Bank AJ. Rosiglitazone improves endothelial function and inflammation but not asymmetric dimethylarginine or oxidative stress in patients with type 2 diabetes mellitus. Vasc Med. 2007;12:311–318. - PubMed
- Esposito K, Ciotola M, Carleo D, Schisano B, Saccomanno F, Sasso FC, Cozzolino D, Assaloni R, Merante D, Ceriello A, Giugliano D. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29:1071–1076. - PubMed
- Wang TD, Chen WJ, Lin JW, Chen MF, Lee YT. Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2004;93:362–365. - PubMed
- Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL061746/HL/NHLBI NIH HHS/United States
- U01 HL061748/HL/NHLBI NIH HHS/United States
- U01 HL061744/HL/NHLBI NIH HHS/United States
- U01 HL063804/HL/NHLBI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- U01 HL038610/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical