Human cytomegalovirus interleukin-10 polarizes monocytes toward a deactivated M2c phenotype to repress host immune responses - PubMed (original) (raw)
Human cytomegalovirus interleukin-10 polarizes monocytes toward a deactivated M2c phenotype to repress host immune responses
Selmir Avdic et al. J Virol. 2013 Sep.
Abstract
Several human cytomegalovirus (HCMV) genes encode products that modulate cellular functions in a manner likely to enhance viral pathogenesis. This includes UL111A, which encodes homologs of human interleukin-10 (hIL-10). Depending upon signals received, monocytes and macrophages become polarized to either classically activated (M1 proinflammatory) or alternatively activated (M2 anti-inflammatory) subsets. Skewing of polarization toward an M2 subset may benefit the virus by limiting the proinflammatory responses to infection, and so we determined whether HCMV-encoded viral IL-10 influenced monocyte polarization. Recombinant viral IL-10 protein polarized CD14(+) monocytes toward an anti-inflammatory M2 subset with an M2c phenotype, as demonstrated by high expression of CD163 and CD14 and suppression of major histocompatibility complex (MHC) class II. Significantly, in the context of productive HCMV infection, viral IL-10 produced by infected cells polarized uninfected monocytes toward an M2c phenotype. We also assessed the impact of viral IL-10 on heme oxygenase 1 (HO-1), which is an enzyme linked with suppression of inflammatory responses. Polarization of monocytes by viral IL-10 resulted in upregulation of HO-1, and inhibition of HO-1 function resulted in a loss of capacity of viral IL-10 to suppress tumor necrosis factor alpha (TNF-α) and IL-1β, implicating HO-1 in viral IL-10-induced suppression of proinflammatory cytokines by M2c monocytes. In addition, a functional consequence of monocytes polarized with viral IL-10 was a decreased capacity to activate CD4(+) T cells. This study identifies a novel role for viral IL-10 in driving M2c polarization, which may limit virus clearance by restricting proinflammatory and CD4(+) T cell responses at sites of infection.
Figures
Fig 1
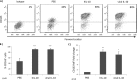
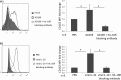
Increased CD163 expression on CD14+ monocytes in response to hIL-10 and viral IL-10 protein treatment. CD14+ monocytes were treated with hIL-10 and viral IL-10 (100 ng/ml) or with PBS (negative control) for 24 h. (A) Representative flow cytometry scatter plots of cells expressing CD163. Graphs depict the percentage of CD163+ cells (B) or fold change (relative to PBS treatment) of CD163 mean fluorescence intensity (MFI) (C). The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Fig 2
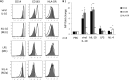
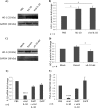
Human monocytes exhibit M2c alternatively activated phenotype in response to viral IL-10 protein treatment. CD14+ monocytes were treated with viral IL-10 (100 ng/ml), hIL-10 (100 ng/ml), lipopolysaccharide (LPS; 1 μg/ml), and hIL-4 (100 ng/ml) or with PBS (negative control) for 24 h. (A) Flow cytometry histograms of human monocytes expressing CD14, CD163, and HLA-DR. Open histograms show expression in treated samples compared to filled histograms that show expression in PBS-treated samples. (B) Graph depicting the fold change (relative to PBS treatment) of CD14, CD163, and HLA-DR mean fluorescence intensity (MFI) for hIL-10-, viral IL-10-, LPS-, and hIL-4-treated monocytes. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test: *, P < 0.05; **, P < 0.01.
Fig 3
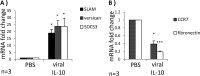
Viral IL-10 upregulates expression of M2c-associated mRNA transcripts in human monocytes. CD14+ monocytes were treated with viral IL-10 (100 ng/ml) or with PBS (negative control) for 24 h. (A) Fold mRNA expression change of M2c-associated transcripts SLAM, versican, and SOCS3 in monocytes treated with viral IL-10 (relative to PBS-treated samples). (B) Fold mRNA expression change of M1-associated transcript CCR7 and M2a-associated transcript fibronectin in monocytes treated with viral IL-10 (relative to PBS-treated samples). The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test: *, P < 0.05; ***, P < 0.001.
Fig 4
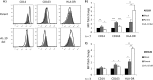
Viral IL-10 expressed during productive HCMV infection polarizes bystander monocytes toward an alternatively activated M2c phenotype. CD14+ monocytes were cultured for 24 h in conditioned supernatants from HFF cultures productively infected with a viral IL-10 deletion virus (vIL-10 del) or parental virus (Parent) or mock infected (Mock) for 24 h. (A) Representative flow cytometry histograms of cells expressing CD14, CD163, and HLA-DR. Open histograms show expression in monocytes cultured with conditioned supernatants from vIL-10 del-infected and parent virus-infected (both in the AD169 backbone) samples compared to filled histograms that show expression in monocytes incubated with conditioned supernatants from mock-infected samples. (B and C) Graphs depicting the mean fluorescence intensity (MFI) fold change of CD14, CD163, and HLA-DR in monocytes cultured with conditioned supernatants from vIL-10 del- and parent virus-infected samples, as indicated (relative to monocytes incubated with conditioned supernatants from mock-infected samples). The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between samples compared to mock infection were determined using a one-tailed, paired Student t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Fig 5
Blocking the human IL-10 receptor on CD14+ monocytes prevents surface CD163 upregulation by viral IL-10. CD14+ monocytes were pretreated with anti-hIL-10R antibody for 2 h prior to incubation for 24 h with conditioned supernatants from HFF cultures productively infected with HCMV strain AD169 (A) or recombinant viral IL-10 (100 ng/ml) (B). Surface CD163 expression with anti-hIL-10R antibody (area under the dashed line) was compared to the surface CD163 expression from the identical treatments but with anti-hIL-10R antibody omitted (A, mock) and treatment with PBS with anti-hIL-10R antibody omitted (B, PBS; negative control). Error bars indicate the standard errors of the means. Significant differences between samples compared to PBS treatment were determined using a one-tailed, paired Student t test (*, P < 0.05).
Fig 6
Heme oxygenase 1 (HO-1) is upregulated in viral IL-10-polarized M2c monocytes and plays a role in viral IL-10-driven suppression of proinflammatory cytokines. (A) Western blot showing HO-1 protein levels in CD14+ monocytes treated with viral IL-10 (100 ng/ml), hIL-10 (100 ng/ml), or PBS (negative control) for 24 h. Expression of GAPDH was used as a protein loading control. (B) The fold change of HO-1 protein expression in monocytes treated with recombinant proteins was determined by densitometry normalized to the expression of GAPDH. (C) Western blot showing HO-1 protein levels in uninfected CD14+ monocytes cultured for 24 h with conditioned supernatants from HFF cultures productively infected with viral IL-10 deletion virus (vIL-10 del), parental virus (Parent), or with supernatants from mock-infected HFFs (Mock). Expression of GAPDH was used as a protein loading control. (D) The fold change of HO-1 protein expression in uninfected monocytes treated with supernatants was determined by densitometry normalized to the expression of GAPDH. Quantitative RT-PCR-based analysis of IL-1β mRNA (E) and TNF-α mRNA (F) in LPS-stimulated human CD14+ monocytes treated with viral IL-10 (100 ng/ml), with or without the HO-1 competitive inhibitor ZnPP (10 nmol/ml) is shown. Graphs depict fold change of mRNA expression relative to cells treated with PBS. Error bars indicate the standard errors of the means. Significant differences were determined using a one-tailed, paired Student t test: *, P < 0.05; ****, P < 0.0001.
Fig 7
CD4+ T cell activation and proliferation are inhibited by viral IL-10-polarized M2c monocytes in a mixed leukocyte reaction. Human CD14+ monocytes polarized with viral IL-10 (100 ng/ml) and hIL-10 (100 ng/ml) or with PBS (no polarization control) were cultured with CD4+ T cells in an allogeneic setting for 24 h prior to examination of an early T cell activation marker, CD69, or for 5 days with CFSE-labeled CD4+ T cells prior to assessment of CD4+ T cell proliferation by flow cytometry. CD4+ T cells with no monocytes added were used as a negative control while CD4+ T cells cultured with PMA-ionomycin were used as a positive control. (A) Representative flow cytometry scatter plots of CD4+ T cells expressing CD69. (B) Graph depicting the percentages of CD69+ CD4+ T cells. (C) Representative flow cytometry histograms showing percentage of proliferating CD4+ T cells. (D) Graph depicting the percentages of proliferating CD4+ T cells. The number of independent biological replicate experiments (n) is shown. Error bars indicate the standard errors of the means. Significant differences between viral IL-10- and hIL-10-treated samples compared to PBS-treated samples were determined using a one-tailed, paired Student t test (*, P < 0.05).
Similar articles
- Human Cytomegalovirus-Encoded Human Interleukin-10 (IL-10) Homolog Amplifies Its Immunomodulatory Potential by Upregulating Human IL-10 in Monocytes.
Avdic S, McSharry BP, Steain M, Poole E, Sinclair J, Abendroth A, Slobedman B. Avdic S, et al. J Virol. 2016 Mar 28;90(8):3819-3827. doi: 10.1128/JVI.03066-15. Print 2016 Apr. J Virol. 2016. PMID: 26792743 Free PMC article. - Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection.
Jenkins C, Garcia W, Godwin MJ, Spencer JV, Stern JL, Abendroth A, Slobedman B. Jenkins C, et al. J Virol. 2008 Apr;82(7):3736-50. doi: 10.1128/JVI.02173-07. Epub 2008 Jan 23. J Virol. 2008. PMID: 18216121 Free PMC article. - Epstein Barr Virus Interleukin 10 Suppresses Anti-inflammatory Phenotype in Human Monocytes.
Jog NR, Chakravarty EF, Guthridge JM, James JA. Jog NR, et al. Front Immunol. 2018 Oct 9;9:2198. doi: 10.3389/fimmu.2018.02198. eCollection 2018. Front Immunol. 2018. PMID: 30356670 Free PMC article. - HCMV Infection and Apoptosis: How Do Monocytes Survive HCMV Infection?
Collins-McMillen D, Chesnokova L, Lee BJ, Fulkerson HL, Brooks R, Mosher BS, Yurochko AD. Collins-McMillen D, et al. Viruses. 2018 Sep 29;10(10):533. doi: 10.3390/v10100533. Viruses. 2018. PMID: 30274264 Free PMC article. Review. - Human cytomegalovirus induction of a unique signalsome during viral entry into monocytes mediates distinct functional changes: a strategy for viral dissemination.
Chan G, Nogalski MT, Stevenson EV, Yurochko AD. Chan G, et al. J Leukoc Biol. 2012 Oct;92(4):743-52. doi: 10.1189/jlb.0112040. Epub 2012 Jun 19. J Leukoc Biol. 2012. PMID: 22715139 Free PMC article. Review.
Cited by
- Cytomegaloviruses and Macrophages-Friends and Foes From Early on?
Baasch S, Ruzsics Z, Henneke P. Baasch S, et al. Front Immunol. 2020 May 12;11:793. doi: 10.3389/fimmu.2020.00793. eCollection 2020. Front Immunol. 2020. PMID: 32477336 Free PMC article. Review. - Human cytomegalovirus manipulation of latently infected cells.
Sinclair JH, Reeves MB. Sinclair JH, et al. Viruses. 2013 Nov 21;5(11):2803-24. doi: 10.3390/v5112803. Viruses. 2013. PMID: 24284875 Free PMC article. Review. - Dynamic Changes of Microglia/Macrophage M1 and M2 Polarization in Theiler's Murine Encephalomyelitis.
Herder V, Iskandar CD, Kegler K, Hansmann F, Elmarabet SA, Khan MA, Kalkuhl A, Deschl U, Baumgärtner W, Ulrich R, Beineke A. Herder V, et al. Brain Pathol. 2015 Nov;25(6):712-23. doi: 10.1111/bpa.12238. Epub 2015 Jan 23. Brain Pathol. 2015. PMID: 25495532 Free PMC article. - IL-10: A Multifunctional Cytokine in Viral Infections.
Rojas JM, Avia M, Martín V, Sevilla N. Rojas JM, et al. J Immunol Res. 2017;2017:6104054. doi: 10.1155/2017/6104054. Epub 2017 Feb 20. J Immunol Res. 2017. PMID: 28316998 Free PMC article. Review. - Loss of IL-10 signaling in macrophages limits bacterial killing driven by prostaglandin E2.
Mukhopadhyay S, Heinz E, Porreca I, Alasoo K, Yeung A, Yang HT, Schwerd T, Forbester JL, Hale C, Agu CA, Choi YH, Rodrigues J, Capitani M, Jostins-Dean L, Thomas DC, Travis S, Gaffney D, Skarnes WC, Thomson N, Uhlig HH, Dougan G, Powrie F. Mukhopadhyay S, et al. J Exp Med. 2020 Feb 3;217(2):e20180649. doi: 10.1084/jem.20180649. J Exp Med. 2020. PMID: 31819956 Free PMC article.
References
- Mocarski ES, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2701–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA
- Goldmacher VS, Bartle LM, Skaletskaya A, Dionne CA, Kedersha NL, Vater CA, Han JW, Lutz RJ, Watanabe S, Cahir McFarland ED, Kieff ED, Mocarski ES, Chittenden T. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U. S. A. 96:12536–12541 - PMC - PubMed
- Cheung AK, Gottlieb DJ, Plachter B, Pepperl-Klindworth S, Avdic S, Cunningham AL, Abendroth A, Slobedman B. 2009. The role of the human cytomegalovirus UL111A gene in downregulating CD4+ T cell recognition of latently infected cells: implications for virus elimination during latency. Blood 114:4128–4137 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials