Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis - PubMed (original) (raw)
Review
Anti-Wolbachia drug discovery and development: safe macrofilaricides for onchocerciasis and lymphatic filariasis
Mark J Taylor et al. Parasitology. 2014 Jan.
Abstract
Anti-Wolbachia therapy delivers safe macrofilaricidal activity with superior therapeutic outcomes compared to all standard anti-filarial treatments, with the added benefit of substantial improvements in clinical pathology. These outcomes can be achieved, in principle, with existing registered drugs, e.g. doxycycline, that are affordable, available to endemic communities and have well known, albeit population-limiting, safety profiles. The key barriers to using doxycycline as an mass drug administration (MDA) strategy for widespread community-based control are the logistics of a relatively lengthy course of treatment (4-6 weeks) and contraindications in children under eight years and pregnancy. Therefore, the primary goal of the anti-Wolbachia (A·WOL) consortium is to find drugs and regimens that reduce the period of treatment from weeks to days (7 days or less), and to find drugs which would be safe in excluded target populations (pregnancy and children). A secondary goal is to refine regimens of existing antibiotics suitable for a more restricted use, prior to the availability of a regimen that is compatible with MDA usage. For example, for use in the event of the emergence of drug-resistance, in individuals with high loiasis co-infection and at risk of severe adverse events (SAE) to ivermectin, or in post-MDA 'endgame scenarios', where test and treat strategies become more cost effective and deliverable.
Figures
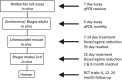
Fig. 1.
Screening funnel developed for A·WOL.
Similar articles
- Short-Course, High-Dose Rifampicin Achieves Wolbachia Depletion Predictive of Curative Outcomes in Preclinical Models of Lymphatic Filariasis and Onchocerciasis.
Aljayyoussi G, Tyrer HE, Ford L, Sjoberg H, Pionnier N, Waterhouse D, Davies J, Gamble J, Metuge H, Cook DAN, Steven A, Sharma R, Guimaraes AF, Clare RH, Cassidy A, Johnston KL, Myhill L, Hayward L, Wanji S, Turner JD, Taylor MJ, Ward SA. Aljayyoussi G, et al. Sci Rep. 2017 Mar 16;7(1):210. doi: 10.1038/s41598-017-00322-5. Sci Rep. 2017. PMID: 28303006 Free PMC article. - Repurposing auranofin as a lead candidate for treatment of lymphatic filariasis and onchocerciasis.
Bulman CA, Bidlow CM, Lustigman S, Cho-Ngwa F, Williams D, Rascón AA Jr, Tricoche N, Samje M, Bell A, Suzuki B, Lim KC, Supakorndej N, Supakorndej P, Wolfe AR, Knudsen GM, Chen S, Wilson C, Ang KH, Arkin M, Gut J, Franklin C, Marcellino C, McKerrow JH, Debnath A, Sakanari JA. Bulman CA, et al. PLoS Negl Trop Dis. 2015 Feb 20;9(2):e0003534. doi: 10.1371/journal.pntd.0003534. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25700363 Free PMC article. - Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: a randomized controlled trial.
Turner JD, Tendongfor N, Esum M, Johnston KL, Langley RS, Ford L, Faragher B, Specht S, Mand S, Hoerauf A, Enyong P, Wanji S, Taylor MJ. Turner JD, et al. PLoS Negl Trop Dis. 2010 Apr 13;4(4):e660. doi: 10.1371/journal.pntd.0000660. PLoS Negl Trop Dis. 2010. PMID: 20405054 Free PMC article. Clinical Trial. - Anti-Wolbachia therapy for onchocerciasis & lymphatic filariasis: Current perspectives.
Wan Sulaiman WA, Kamtchum-Tatuene J, Mohamed MH, Ramachandran V, Ching SM, Sazlly Lim SM, Hashim HZ, Inche Mat LN, Hoo FK, Basri H. Wan Sulaiman WA, et al. Indian J Med Res. 2019 Jun;149(6):706-714. doi: 10.4103/ijmr.IJMR_454_17. Indian J Med Res. 2019. PMID: 31496523 Free PMC article. Review. - Filariasis in Africa--treatment challenges and prospects.
Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S. Hoerauf A, et al. Clin Microbiol Infect. 2011 Jul;17(7):977-85. doi: 10.1111/j.1469-0691.2011.03586.x. Clin Microbiol Infect. 2011. PMID: 21722251 Review.
Cited by
- Microbe Profile: Wolbachia: a sex selector, a viral protector and a target to treat filarial nematodes.
Taylor MJ, Bordenstein SR, Slatko B. Taylor MJ, et al. Microbiology (Reading). 2018 Nov;164(11):1345-1347. doi: 10.1099/mic.0.000724. Epub 2018 Oct 12. Microbiology (Reading). 2018. PMID: 30311871 Free PMC article. - Host genetic backgrounds: the key to determining parasite-host adaptation.
Ye C, Zhang L, Tang L, Duan Y, Liu J, Zhou H. Ye C, et al. Front Cell Infect Microbiol. 2023 Aug 10;13:1228206. doi: 10.3389/fcimb.2023.1228206. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37637465 Free PMC article. Review. - Efficacy of three-week oxytetracycline or rifampin monotherapy compared with a combination regimen against the filarial nematode Onchocerca ochengi.
Bah GS, Ward EL, Srivastava A, Trees AJ, Tanya VN, Makepeace BL. Bah GS, et al. Antimicrob Agents Chemother. 2014;58(2):801-10. doi: 10.1128/AAC.01995-13. Epub 2013 Nov 18. Antimicrob Agents Chemother. 2014. PMID: 24247133 Free PMC article. - Fit for purpose: do we have the right tools to sustain NTD elimination?
Reimer LJ, Adams ER, Paine MJ, Ranson H, Coleman M, Thomsen EK, MacPherson EE, Hollingsworth TD, Kelly-Hope LA, Bockarie MJ, Ford L, Harrison RA, Stothard JR, Taylor MJ, Hamon N, Torr SJ. Reimer LJ, et al. BMC Proc. 2015 Dec 18;9(Suppl 10):S5. doi: 10.1186/1753-6561-9-S10-S5. eCollection 2015. BMC Proc. 2015. PMID: 28281703 Free PMC article. - Reciprocal Interactions between Nematodes and Their Microbial Environments.
Midha A, Schlosser J, Hartmann S. Midha A, et al. Front Cell Infect Microbiol. 2017 Apr 27;7:144. doi: 10.3389/fcimb.2017.00144. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28497029 Free PMC article. Review.
References
- Ash L. R. and Riley J. M. (1970). Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus, with notes on infections in other rodents. Journal of Parasitology 56, 969–973 - PubMed
- Coffeng L. E., Stolk W. A., Zouré H. G., Veerman J. L., Agblewonu K. B., Murdoch M. E., Noma M., Fobi G., Richardus J. H., Bundy D. A., Habbema D., de Vlas S. J. and Amazigo U. V. (2013). African Programme For Onchocerciasis Control 1995–2015: model-estimated health impact and cost. PLoS Neglected Tropical Diseases 7, e2032.10.1371/journal.pntd.0002032 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical