Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis - PubMed (original) (raw)
. 2013 Sep;89(6):1084-98.
doi: 10.1111/mmi.12335. Epub 2013 Aug 4.
Affiliations
- PMID: 23869552
- PMCID: PMC3817527
- DOI: 10.1111/mmi.12335
Free PMC article
Differentiated roles for MreB-actin isologues and autolytic enzymes in Bacillus subtilis morphogenesis
Patricia Domínguez-Cuevas et al. Mol Microbiol. 2013 Sep.
Free PMC article
Abstract
Cell morphogenesis in most bacteria is governed by spatiotemporal growth regulation of the peptidoglycan cell wall layer. Much is known about peptidoglycan synthesis but regulation of its turnover by hydrolytic enzymes is much less well understood. Bacillus subtilis has a multitude of such enzymes. Two of the best characterized are CwlO and LytE: cells lacking both enzymes have a lethal block in cell elongation. Here we show that activity of CwlO is regulated by an ABC transporter, FtsEX, which is required for cell elongation, unlike cell division as in Escherichia coli. Actin-like MreB proteins are thought to play a key role in orchestrating cell wall morphogenesis. B. subtilis has three MreB isologues with partially differentiated functions. We now show that the three MreB isologues have differential roles in regulation of the CwlO and LytE systems and that autolysins control different aspects of cell morphogenesis. The results add major autolytic activities to the growing list of functions controlled by MreB isologues in bacteria and provide new insights into the different specialized functions of essential cell wall autolysins.
© 2013 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Figures
Fig. 1
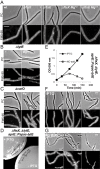
FtsEX mutants are similar to Δ_cwlO_ and synthetic lethal with Δ_lytE_. A. Cell morphologies of typical fields of wild-type B. subtilis strain 168, Δ_ftsX_ ( 4501 ) and Δ_ftsE_ ( 4503 ) mutant strains growing in a NA plates or in NA plates with supplement of 20 mM Mg2+, as indicated. Scale bar represents 5 μm. B and C. Cell morphologies of typical fields of strains PDC464 (Δ_lytE_::cat) and PDC463 (Δ_cwlO_ :: spec) cultured on NA plates in the presence or absence of Mg2+ as indicated. Scale bar represents 5 μm. D. Growth of strain PDC492 (Δ_ftsX_ :: neo Δ_lytE_ :: cat aprE :: Pspac - LytE) on NA plates with or without 0.5 mM IPTG. E. Growth of strain PDC492 on LB liquid medium in the presence or absence of IPTG. Growth curves (IPTG 0.5 mM, closed symbols; no addition, open symbols). F and G. Effect of LytE depletion on cell morphology. Phase-contrast micrographs and the corresponding membrane staining images were taken at the indicated times during the growth curves in (E). (F) 0.5 mM IPTG added; (G) no IPTG addition. Scale bar represents 5 μm.
Fig. 2
Bacterial two-hybrid analysis of FtsEX protein interactions. A. Bacterial two-hybrid analysis of interaction between FtsE and FtsX. B. Bacterial two-hybrid analysis of interaction with FtsE and FtsX. Escherichia coli strain BTH101 was co-transformed with two-hybrid vector plasmids (pUT18 and pKT25) expressing C-terminal fusions of the cyaA T18 domain to ftsE, ftsX and ftsEX, and N-terminal fusions of cyaA T25 domain to various genes, as indicated. Transformants were spotted onto nutrient agar plates containing X-Gal and incubated at 30°C for 40 h. Blue colouration indicates a positive interaction.
Fig. 3
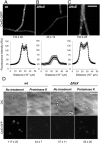
CwlO localizes at the cell membrane in an FtsX-dependent manner. A–C. Epifluorescence microscopy of strains expressing the fluorescent fusion amyE :: Pxyl - cwlO - gfpsf. The different panels correspond to (A) strain PDC528 (Bs168CA wprA :: hyg, epr :: tet amyE :: Pxyl - cwlO - gfpsf) and isogenic strains (B) PDC560 (Δ_ftsX_), (C) PDC594 (Δ_ftsE_), as indicated. Scale bar represents 5 μm. Fluorescent images were taken with the same acquisition settings and exposure times, with the maximum averaged value of quantified fluorescence intensity over the lateral wall of the cells (see Experimental procedures) indicated below. Lower panels: Profiles of fluorescence intensity corresponding to strains in (A)–(C) respectively. Averaged fluorescence intensity quantified in segments of equal size across the cell's longitudinal axis. The _y_-axis represents fluorescence intensity (relative units, RU), while the x_-axis represents distance (10−1 μm). Error bars represent standard deviation of fluorescent intensity measurements. D. Cells of strains PDC528 (wt, left panels) and PDC560 (Δ_ftsX :: neo, right panels) were grown in CH media in the presence of 0.5% xylose and protoplasted (see Experimental procedures). Images show the relative fluorescence intensity from CwlO–GFPsf protoplasts treated with proteinase K or not, as indicated. Maximum averaged value of quantified fluorescence intensities across the cells are shown below together with calculated standard deviations. Scale bar: 5 μm.
Fig. 4
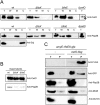
CwlO and FtsX form part of a protein complex in the cell membrane. A. Reduction in CwlO membrane fraction levels in the absence of FtsX. Fractionation of wt, Δ_ftsE_ and Δ_ftsX_ mutants. Total fraction from Δ_cwlO_ mutant strain was analysed to discard unspecific bands. Solid black arrow indicates CwlO band after Western blotting. Dashed arrow indicates unspecific band. Lower panels correspond to cell fractionation controls using polyclonal antibodies against Pbp2B and Soj membrane and cytosolic proteins respectively. B. CwlO is detected predominantly in the supernatant fraction. C. Pull-down of CwlO-FLAG complexes in membranes of exponentially growing cells treated with formaldehyde (described in Experimental procedures). Bands on the Western blots were detected with anti-FLAG, anti-GFP, anti-Pbp2B, anti-MreB and anti-DivIVA antibodies, as indicated. Left lanes correspond to the control strain PDC528 were CwlO remains untagged, while expresses an FtsX–GFP fusion. Right lanes correspond to the strain PDC612 that coexpresses CwlO–FLAG and FtsX–GFP fusion proteins. T, total-cell extract prior cross-linking; P, heated pull-down fraction; the experiment was performed three times with similar results.
Fig. 5
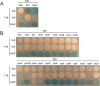
CwlO activity depends on the Mbl actin homologue. A–C. Growth of strains B-only, BL-only and BH-only (a/c), respectively, and its derivatives PDC664, PDC643 and PDC659 (b/d) on NA 20 mM Mg2+, supplemented with appropriate concentration of IPTG in each case (Kawai et al., 2009a), in the presence (a–b) or absence of xylose (c–d), as indicated. D–F. Cell morphologies of typical fields of strains in (A)–(C) respectively. Phase-contrast and membrane-stained fluorescent images (FM5-95) of parental strains (a) and its derivatives in the presence (b) or absence (d) of xylose for 3 h at 37°C. G–I. Growth of strains B-only, BL-only and BH-only (a/c), respectively, and its derivatives (Δ_lytE_ :: spec, aprE :: Pxyl - lytE) PDC688, PDC678 and PDC697 (b/d) on NA 20 mM Mg2+, supplemented with appropriate concentration of IPTG in each case (Kawai et al., 2009a), in the presence (a–b) or absence of xylose (c–d). J–L. Cell morphologies of typical fields of strains in (G)–(I) respectively. Phase-contrast and membrane-stained fluorescent images (FM5-95) of parental strains (a) and its derivatives in the presence (b) or absence (d) of xylose for 3 h at 37°C.
Fig. 6
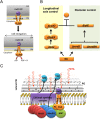
Model for actin cytoskeleton function in co-ordination of CW hydrolytic activities CwlO and LytE during cell elongation. A. Schematic representation of the FtsEX ATPase cycle during activation of the CwlO CW hydrolase activity in B. subtilis. FtsEX is shown in the model as a tetramer, although other stoichiometries are possible on the basis of the two-hybrid data. B. Two distinct pathways for CW hydrolytic activity at the lateral cell wall in B. subtilis. C. Co-ordination by the actin cytoskeleton to ensure the balance between cell wall synthesis and hydrolysis during cell elongation. See text for further details.
Similar articles
- FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis.
Meisner J, Montero Llopis P, Sham LT, Garner E, Bernhardt TG, Rudner DZ. Meisner J, et al. Mol Microbiol. 2013 Sep;89(6):1069-83. doi: 10.1111/mmi.12330. Epub 2013 Aug 1. Mol Microbiol. 2013. PMID: 23855774 Free PMC article. - The WalR-WalK Signaling Pathway Modulates the Activities of both CwlO and LytE through Control of the Peptidoglycan Deacetylase PdaC in Bacillus subtilis.
Dobihal GS, Flores-Kim J, Roney IJ, Wang X, Rudner DZ. Dobihal GS, et al. J Bacteriol. 2022 Feb 15;204(2):e0053321. doi: 10.1128/JB.00533-21. Epub 2021 Dec 6. J Bacteriol. 2022. PMID: 34871030 Free PMC article. - SweC and SweD are essential co-factors of the FtsEX-CwlO cell wall hydrolase complex in Bacillus subtilis.
Brunet YR, Wang X, Rudner DZ. Brunet YR, et al. PLoS Genet. 2019 Aug 22;15(8):e1008296. doi: 10.1371/journal.pgen.1008296. eCollection 2019 Aug. PLoS Genet. 2019. PMID: 31437162 Free PMC article. - Autolysins of Bacillus subtilis: multiple enzymes with multiple functions.
Smith TJ, Blackman SA, Foster SJ. Smith TJ, et al. Microbiology (Reading). 2000 Feb;146 ( Pt 2):249-262. doi: 10.1099/00221287-146-2-249. Microbiology (Reading). 2000. PMID: 10708363 Review. No abstract available. - Roles of FtsEX in cell division.
Pichoff S, Du S, Lutkenhaus J. Pichoff S, et al. Res Microbiol. 2019 Nov-Dec;170(8):374-380. doi: 10.1016/j.resmic.2019.07.003. Epub 2019 Aug 1. Res Microbiol. 2019. PMID: 31376483 Free PMC article. Review.
Cited by
- A highly unstable transcript makes CwlO D,L-endopeptidase expression responsive to growth conditions in Bacillus subtilis.
Noone D, Salzberg LI, Botella E, Bäsell K, Becher D, Antelmann H, Devine KM. Noone D, et al. J Bacteriol. 2014 Jan;196(2):237-47. doi: 10.1128/JB.00986-13. Epub 2013 Oct 25. J Bacteriol. 2014. PMID: 24163346 Free PMC article. - Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes.
Do T, Page JE, Walker S. Do T, et al. J Biol Chem. 2020 Mar 6;295(10):3347-3361. doi: 10.1074/jbc.REV119.010155. Epub 2020 Jan 23. J Biol Chem. 2020. PMID: 31974163 Free PMC article. Review. - Bacterial symbiont subpopulations have different roles in a deep-sea symbiosis.
Hinzke T, Kleiner M, Meister M, Schlüter R, Hentschker C, Pané-Farré J, Hildebrandt P, Felbeck H, Sievert SM, Bonn F, Völker U, Becher D, Schweder T, Markert S. Hinzke T, et al. Elife. 2021 Jan 6;10:e58371. doi: 10.7554/eLife.58371. Elife. 2021. PMID: 33404502 Free PMC article. - Evidence that glycopolymer transferases promote peptidoglycan hydrolysis in Bacillus subtilis.
Cohen JD. Cohen JD. bioRxiv [Preprint]. 2025 Mar 11:2025.02.26.640348. doi: 10.1101/2025.02.26.640348. bioRxiv. 2025. PMID: 40060662 Free PMC article. Preprint. - Structural Characterization of the Essential Cell Division Protein FtsE and Its Interaction with FtsX in Streptococcus pneumoniae.
Alcorlo M, Straume D, Lutkenhaus J, Håvarstein LS, Hermoso JA. Alcorlo M, et al. mBio. 2020 Sep 1;11(5):e01488-20. doi: 10.1128/mBio.01488-20. mBio. 2020. PMID: 32873757 Free PMC article.
References
- Bhavsar AP, Brown ED. Cell wall assembly in Bacillus subtilis: how spirals and spaces challenge paradigms. Mol Microbiol. 2006;60:1077–1090. - PubMed
- Bisicchia P, Noone D, Lioliou E, Howell A, Quigley S, Jensen T, et al. The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis. Mol Microbiol. 2007;65:180–200. - PubMed
- den Blaauwen T, de Pedro MA, Nguyen-Disteche M, Ayala JA. Morphogenesis of rod-shaped sacculi. FEMS Microbiol Rev. 2008;32:321–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases