Noncanonical autophagy promotes the visual cycle - PubMed (original) (raw)
Noncanonical autophagy promotes the visual cycle
Ji-Young Kim et al. Cell. 2013.
Erratum in
- Cell. 2013 Oct 24;155(3):725-6
Abstract
Phagocytosis and degradation of photoreceptor outer segments (POS) by retinal pigment epithelium (RPE) is fundamental to vision. Autophagy is also responsible for bulk degradation of cellular components, but its role in POS degradation is not well understood. We report that the morning burst of RPE phagocytosis coincided with the enzymatic conversion of autophagy protein LC3 to its lipidated form. LC3 associated with single-membrane phagosomes containing engulfed POS in an Atg5-dependent manner that required Beclin1, but not the autophagy preinitiation complex. The importance of this process was verified in mice with Atg5-deficient RPE cells that showed evidence of disrupted lysosomal processing. These mice also exhibited decreased photoreceptor responses to light stimuli and decreased chromophore levels that were restored with exogenous retinoid supplementation. These results establish that the interplay of phagocytosis and autophagy within the RPE is required for both POS degradation and the maintenance of retinoid levels to support vision.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
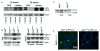
Figure 1. Autophagy in the mouse eye
A. RPE and retinae were isolated from 12 and 28 week old C57BL/6J mice at 7:00 AM and 7:00 PM. Western blotting was performed to determine the amount of the non-lipidated (LC3-I) and lipidated (LC3-II) LC3 present. β-actin was used as the loading control. B. RPE and retinae were isolated at 8:00 AM from control (Atg5f/f) and ATG5ΔRPE mice at 8 and 16 weeks. Western blotting was performed to determine the amount of Atg5 and LC3. β-actin was used as the loading control. C. Posterior cups were harvested at 8 AM from Atg5ΔRPE and littermate controls. Retinae were removed and RPE flat mounts were prepared and then placed in Chloroquine (CLQ) for 1 h. RPE were isolated and western blotting for LC3 was performed. D. Atg5ΔRPE mice were crossed to the GFP-LC3 reporter mouse and RPE flat mounts (harvested at 8:00 AM) were examined for GFP-LC3 punctae.
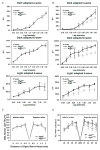
Figure 2. ERG analysis of the Atg5ΔRPE mouse
ERGs were performed on Atg5ΔRPE and littermate control mice at 12 weeks (A) and 16 weeks (B). Each point represents the average of 5 mice. C. ONL counts were performed on control and Atg5ΔRPE retina in the superior and inferior direction from H&E sections as described in the Materials and Methods. No significant differences were noted (p<.05). D. The number of cones was enumerated from PNA stained frozen cross section in 4 zones in the superior and inferior direction from the optic nerve head (ONH). No significant differences were noted (p<.05).
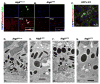
Figure 3. Atg5 and LC3 associate with phagocytosed outer segments
RPE flat mounts were prepared at 8:00 AM from 16 week old (A) littermate control (Atgf/wt;cre+) and (B) Atg5ΔRPE mice, stained with anti-rhodopsin (red) and anti-Atg5 (green), and visualized by confocal microscopy. Nuclei were visualized by DAPI staining (blue). Reconstructed confocal Z-stack images were rotated 90° to visualize Atg5/rhodopsin association in control (Panel A, merged image, inset) and Atg5ΔRPE RPE cells (Panel B, merged image, inset). Bar = 20μm. C. RPE flat mounts prepared from GFP-LC3 mice were stained with anti-rhodopsin (red) to visualize association of LC3 and the POS. DAPI staining was used to visualize the nuclei (blue). D. TEM of a representative RPE from littermate control mice showing electron dense single membrane phagosomes (arrows). E–G. TEM images of a representative Atg5ΔRPE RPE cells showing degenerating phagosomes (arrowheads). Scale bar =1 μm. See also Figure S1
Figure 4. Components of the autophagy pathway promote LC3 translocation to the outer segment containing phagosome
GFP-LC3+ RPE-J cells were transfected with scrambled (Scr) (Panels A–E) or Atg5, Beclin1, Fip200, Ulk1, or Atg13 (panels A, B, C, D, E, respectively) siRNA oligonucleotides and fed labeled POS (red) or treated with rapamycin. Representative images are shown. Inset images in lower panels indicate individual phagosomes denoted by arrows or arrowheads. The percentage (%) of GFP-LC3+ phagosomes (arrows and arrowheads) was quantified following POS feeding from time-lapse captured images (Bar graphs). The number of GFP-LC3+ punctae/cell was quantified 24 h following rapamycin treatment (bar graphs). *** = p< .05. F. Representative electron micrographic image of a phagosome in an RPE-J cell taken 6 h following POS feeding demonstrating a single membrane structure (black arrows). Scale bars = 10 μm. See also Figure S2.
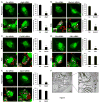
Figure 5. LC3 associated phagocytosis facilitates phagosome maturation and degradation of engulfed POS by RPE cells
GFP-LC3+ RPE-J cells were transfected with A. scrambled (Scr) or B. Atg5 siRNA oligonucleotides and fed labeled POS (red). Cells were stained for Lamp1, Lamp2, or Cathepsin D and co-localization was assessed by confocal microscopy. Representative images (of >25 cells visualized) are shown (taken at 16 h post-POS feeding). C. RPE-J cells were transfected with scrambled (Scr) or Atg5 siRNA oligonucleotides and fed POS. Cells were harvested at 1, 2, 6 h and western blotting was performed for Lamp1, Lamp2, and Cathepsin D. D. RPE-J cells were transfected with scrambled (Scr) or Atg5 siRNA oligonucleotides and fed POS. At 1, 3 and 6 h post feeding confocal images were captured; z- stack images were reconstructed and rotated 90°. In the “No POS” group a nuclear stain (Draq5) was used to visualize nuclei (green). Images at 1, 3 and 6 h do not show the nuclear stain but the position of the nuclei in the cells is indicated by the dotted line oval. The basal surface of the RPE is denoted (dotted straight line). Scale bars = 10μm. See also Figure S3.
Figure 6. Autophagy deficient Ulk1−/− mice have normal vision and demonstrate association of Atg5 with phagocytosed POS
A. Western blots for LC3 and p62 in the RPE were performed on 16 weeks Ulk1−/− and Ulk1+/− mice. B. ERG recording on 16 week old Ulk1−/− and Ulk1+/− mice demonstrating that scotopic and photopic responses were normal. C–D. Association of Atg5 and POS 16 week old Ulk1−/− and Ulk1+/− mice as detected by co-stains for Atg5 and rhodopsin on RPE flat mounts. E–F. Reconstructed confocal Z-stack images were rotated 90° to visualize Atg5/rhodopsin association in Ulk1−/− and Ulk1+/− mice.
Figure 7. Delivery of retinoids restores visual function
A. ERG recordings were performed on Atg5ΔRPE (n=8) and littermate control mice (n=8). B. After 1 week of recovery in a normal light cycle environment each mouse received a single intraperitoneal injection of 0.25 mg of 9-cis RAL, a functional analog of 11-cis RAL. The animals were then dark-adapted for 24 h followed by scotopic and photopic ERG recordings. C. Retinoids were extracted from eyecups of 20- week old Atg5ΔRPE (n=4, gray bar) and Atg5f/f littermate control (n=5, black bar) mice dark- adapted for 24 h prior to the experiments. RAL = retinal; RE = retinyl-esters. See also Figure S4.
Comment in
- Cell biology: Recycling in sight.
Boya P, Codogno P. Boya P, et al. Nature. 2013 Sep 5;501(7465):40-2. doi: 10.1038/501040a. Nature. 2013. PMID: 24005411 No abstract available.
Similar articles
- RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye.
Muniz-Feliciano L, Doggett TA, Zhou Z, Ferguson TA. Muniz-Feliciano L, et al. Autophagy. 2017;13(12):2072-2085. doi: 10.1080/15548627.2017.1380124. Autophagy. 2017. PMID: 28933590 Free PMC article. - The Contribution of Melanoregulin to Microtubule-Associated Protein 1 Light Chain 3 (LC3) Associated Phagocytosis in Retinal Pigment Epithelium.
Frost LS, Lopes VS, Bragin A, Reyes-Reveles J, Brancato J, Cohen A, Mitchell CH, Williams DS, Boesze-Battaglia K. Frost LS, et al. Mol Neurobiol. 2015 Dec;52(3):1135-1151. doi: 10.1007/s12035-014-8920-5. Epub 2014 Oct 10. Mol Neurobiol. 2015. PMID: 25301234 Free PMC article. - Live Imaging of LysoTracker-Labelled Phagolysosomes Tracks Diurnal Phagocytosis of Photoreceptor Outer Segment Fragments in Rat RPE Tissue Ex Vivo.
Mao Y, Finnemann SC. Mao Y, et al. Adv Exp Med Biol. 2016;854:717-23. doi: 10.1007/978-3-319-17121-0_95. Adv Exp Med Biol. 2016. PMID: 26427480 Free PMC article. - Autophagy in the retinal pigment epithelium: a new vision and future challenges.
Intartaglia D, Giamundo G, Conte I. Intartaglia D, et al. FEBS J. 2022 Nov;289(22):7199-7212. doi: 10.1111/febs.16018. Epub 2021 May 31. FEBS J. 2022. PMID: 33993621 Free PMC article. Review. - Safely removing cell debris with LC3-associated phagocytosis.
Fazeli G, Wehman AM. Fazeli G, et al. Biol Cell. 2017 Oct;109(10):355-363. doi: 10.1111/boc.201700028. Epub 2017 Aug 25. Biol Cell. 2017. PMID: 28755428 Review.
Cited by
- Lack of Acid Sphingomyelinase Induces Age-Related Retinal Degeneration.
Wu BX, Fan J, Boyer NP, Jenkins RW, Koutalos Y, Hannun YA, Crosson CE. Wu BX, et al. PLoS One. 2015 Jul 13;10(7):e0133032. doi: 10.1371/journal.pone.0133032. eCollection 2015. PLoS One. 2015. PMID: 26168297 Free PMC article. - LAP: the protector against autoimmunity.
Bandyopadhyay U, Overholtzer M. Bandyopadhyay U, et al. Cell Res. 2016 Aug;26(8):865-6. doi: 10.1038/cr.2016.70. Epub 2016 Jun 14. Cell Res. 2016. PMID: 27297234 Free PMC article. - Network biology analysis of P23H rhodopsin interactome identifies protein and mRNA quality control mechanisms.
Kim K, Safarta LA, Chiang WJ, Coppinger JA, Lee EJ, Lin JH. Kim K, et al. Sci Rep. 2022 Oct 18;12(1):17405. doi: 10.1038/s41598-022-22316-8. Sci Rep. 2022. PMID: 36258031 Free PMC article. - Lipid Droplet Accumulation Promotes RPE Dysfunction.
Yako T, Otsu W, Nakamura S, Shimazawa M, Hara H. Yako T, et al. Int J Mol Sci. 2022 Feb 4;23(3):1790. doi: 10.3390/ijms23031790. Int J Mol Sci. 2022. PMID: 35163712 Free PMC article. - LAP it up, fuzz ball: a short history of LC3-associated phagocytosis.
Martinez J. Martinez J. Curr Opin Immunol. 2018 Dec;55:54-61. doi: 10.1016/j.coi.2018.09.011. Epub 2018 Oct 2. Curr Opin Immunol. 2018. PMID: 30286399 Free PMC article. Review.
References
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. - PubMed
- Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J Histochem Cytochem. 1993;41:253–263. - PubMed
- Cuervo AM, Bergamini E, Brunk UT, Droge W, French M, Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. - PubMed
- Davidson AE, Millar ID, Burgess-Mullan R, Maher GJ, Urquhart JE, Brown PD, Black GC, Manson FD. Functional characterization of bestrophin-1 missense mutations associated with autosomal recessive bestrophinopathy. Invest Ophthalmol Vis Sci. 2011;52:3730–3736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI40646/AI/NIAID NIH HHS/United States
- R01 EY019065/EY/NEI NIH HHS/United States
- EY015570/EY/NEI NIH HHS/United States
- EY02687/EY/NEI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- R01 AI040646/AI/NIAID NIH HHS/United States
- P30 EY002687/EY/NEI NIH HHS/United States
- R01 EY015570/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases