Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase - PubMed (original) (raw)
Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase
Yung-Wei Pan et al. Neurobiol Learn Mem. 2013 Oct.
Abstract
Adult neurogenesis occurs in two discrete regions of the adult mammalian brain, the subgranular zone (SGZ) of the dentate gyrus (DG) and the subventricular zone (SVZ) along the lateral ventricles. Signaling mechanisms regulating adult neurogenesis in the SGZ are currently an active area of investigation. Adult-born neurons in the DG functionally integrate into the hippocampal circuitry and form functional synapses, suggesting a role for these neurons in hippocampus-dependent memory formation. Although results from earlier behavioral studies addressing this issue were inconsistent, recent advances in conditional gene targeting technology, viral injection and optogenetic approaches have provided convincing evidence supporting a role for adult-born neurons in the more challenging forms of hippocampus-dependent learning and memory. Here, we briefly summarize these recent studies with a focus on extra signal-regulated kinase (ERK) 5, a MAP kinase whose expression in the adult brain is restricted to the neurogenic regions including the SGZ and SVZ. We review evidence identifying ERK5 as a novel endogenous signaling pathway that regulates the pro-neural transcription factor Neurogenin 2, is activated by neurotrophins and is critical for adult neurogenesis. We discuss studies demonstrating that specific deletion of ERK5 in the adult neurogenic regions impairs several forms of hippocampus-dependent memory formation in mice. These include contextual fear memory extinction, the establishment and maintenance of remote contextual fear memory, and several other challenging forms of hippocampus-dependent memory formation including 48h memory for novel object recognition, contextual fear memory established by a weak foot shock, pattern separation, and reversal of spatial learning and memory. We also briefly discuss current evidence that increasing adult neurogenesis, by small molecules or genetic manipulation, improves memory formation and long-term memory.
Keywords: Adult neurogenesis; Learning and memory; MAPK; Signal transduction.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
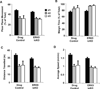
Figure 1. Motor behavior and habituation in the open field assay is not affected in ERK5 icKO animals compared to the drug control mice
Adult male nestin-creER™/ERK5loxP/loxP and ERK5loxP/loxP littermate mice (10–12 weeks old) were treated with tamoxifen as in (Pan et al., 2012a) and designated ERK5 icKO or drug control respectively. Mice were exposed to the open field assay for three consecutive days to examine habituation to a novel environment. (A) Total floor plane activity on day 1 (d1), d2, d3. (B) Total amount of time spent along the margin of the open field chamber. (C) Total distance traveled during each exposure to the open field chamber. (D) Average traveling speed during each exposure. Data represent mean ± SEM of two independent experiments with n ≥ 10 animals per treatment group per experiment.
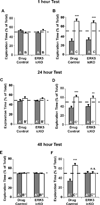
Figure 2. Drug control animals retain, while ERK5 icKO mice lack memory in the novel object recognition assay tested 48 h after training
As described in (Pan et al., 2012a), animals were trained for two objects (A and B) during the training session for 5 min (A–B) or 10 min (C–F). Following an interval of 1 h, 24 h, or 48 h, object B was replaced with object C and animals were permitted to explore both object A (familiar) and object C (novel) in the testing session. Total exploration time of each object was recorded identically during the training (A, C, E) and testing sessions (B, D, F). A unique set of objects A–C was used for each time point tested. Memory for the familiar object A is manifested as more time spent in exploring the novel object C than the familiar object A. (A, B) ERK5 icKO mice have normal 1 h memory. (C, D) ERK5 icKO mice have normal 24 h memory. (E, F) ERK5 icKO mice have no memory 48 h post-training. Data represent mean exploration time ± SEM from three independent experiments with n ≥ 8 animals per treatment group per experiment. n.s., not significant; **, p < 0.01; ***, p < 0.001.
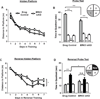
Figure 3. ERK5 icKO animals are deficient in acquiring newer spatial information during reversal training in the Morris water maze
(A) As described in (Pan et al., 2012a), mice were subjected to 8 d of training (4 sessions/day, 30 min inter-session interval, from d1 to d8) in the hidden platform Morris water maze. Target (in quadrant #1) acquisition, quantified as swim distance to the hidden platform, was similar in drug control and ERK5 icKO mice, demonstrating similar spatial learning. (B) A probe test was conducted 24 h later, which demonstrated that drug control and ERK5 icKO mice both acquired spatial memory for the location of the hidden platform. (C) Mice were then subjected to 7 d of reversal training (4 sessions/day, 30 min inter-session interval) where the escape hidden platform was relocated to the opposite quadrant (quadrant #3) of the water maze. ERK5 icKO mice swam longer distances to find the hidden platform in the newer location, suggesting impaired learning of newer spatial information. (D) ERK5 icKO animals are unable to differentiate between the new and old locations of the escape platform in the reversal probe test, conducted 24 h after reversal hidden platform training. Data represent mean ± SEM from two independent experiments with n ≥ 10 animals per treatment group per experiment. n.s., not significant; *, p < 0.05; ***, p < 0.001.
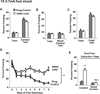
Figure 4. ERK5 icKO mice are deficient in contextual fear memory extinction
As described in (Pan et al., 2012a), all animals were trained in the contextual fear conditioning assay using a single 0.7 mA foot shock lasting 2 s. A 30 s tone was presented prior to foot shock delivery and co-terminated with the foot shock. (A) Drug control and ERK5 icKO animals have similar levels of contextual fear memory 24 h after training. (B) Fear memory is context specific since neither drug control nor ERK5 icKO mice froze when placed in a novel context 24 h after training. (C) Cued-fear response is similar between drug control and ERK5 icKO animals when tested 24 h after training. (D) Fear extinction is impaired in ERK5 icKO animals compared to drug controls. Day 0: the day of contextual fear testing. Day 1: the first day animals were subjected to fear extinction training. (E) Following fear extinction training, mice were placed into a novel context on Day 9. Both drug control and ERK5 icKO animals froze minimally when placed in the novel context, suggesting that the persistent fear memory associated with ERK5 icKO mice is context-specific behavior. Data represent mean freezing behavior ± SEM from two independent experiments with n ≥ 8 animals per treatment group per experiment. n.s., not significant; ***, p < 0.001.
Figure 5. ERK5 icKO animals have reduced contextual fear memory when trained using a more challenging training paradigm
As described in (Pan et al., 2012a), animals were trained using a weaker electric foot shock (0.3 mA) delivered 3 consecutive times. Each foot shock was paired with a tone, which co-terminated with the foot shock. (A) Freezing behavior was monitored in the shocking context 24 h after training. ERK5 icKO mice froze less compared with drug control animals. (B) This deficit was context-specific since both groups of animals did not freeze when exposed to a novel context 24 h after training. (C) Cued-fear conditioning was also not affected in either group of mice when tested 24 h after training. Data represent mean freezing behavior ± SEM from two independent experiments with n ≥ 6 animals per treatment group per experiment. ***, p < 0.001.
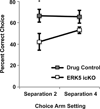
Figure 6. ERK5 icKO mice show impaired pattern separation
As described in (Pan et al., 2012a), mice were trained in the delayed-non-matching-to-place radial arm maze assay as a test for pattern separation (Pan et al., 2012a). When the food reward was moved 180° from the original sample arm location (Separation 4), ERK5 icKO performed nearly as well as drug control animals in acquiring this spatial memory. However, ERK5 icKO mice were unable to distinguish between the sample and test arms when the food reward was placed in the test arm 90° from the original sample arm location (Separation 2), which is a more difficult test than separation 4. Data is from the last day of a 5 d experimental paradigm and represents mean ± SEM with n ≥ 10 per treatment group. *, p < 0.05.
Figure 7. Remote memory is impaired in ERK5 icKO mice when tested in the passive avoidance assay
As described in (Pan et al., 2012a), mice were trained in the passive avoidance assay by placing animals in the light side of a light/dark box. The light/dark chambers are separated by a small trap door. When mice entered the dark side of the box, the trap door immediately closed and a single 0.7 mA foot shock was delivered for 2 s. Following a 1 min memory acquisition period, mice were removed from the dark side of the box. Mice were placed back to the light side of the training box 24 h later to assess short-term, 24 h memory for the aversive stimulus, manifested as increased latency to cross over into the dark side. No deficits exist between drug control and ERK5 icKO animals at 24 h. Mice were then returned to their home cages. At 21 d post-training, animals were again placed back to the light side of the box to assess for remote memory. Unlike drug control mice, ERK5 icKO animals had no remote memory. Data represent mean crossover latency ± SEM with n ≥ 10 per treatment group. ***, p < 0.001.
Similar articles
- The maintenance of established remote contextual fear memory requires ERK5 MAP kinase and ongoing adult neurogenesis in the hippocampus.
Pan YW, Storm DR, Xia Z. Pan YW, et al. PLoS One. 2012;7(11):e50455. doi: 10.1371/journal.pone.0050455. Epub 2012 Nov 26. PLoS One. 2012. PMID: 23189204 Free PMC article. - Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis.
Pan YW, Zou J, Wang W, Sakagami H, Garelick MG, Abel G, Kuo CT, Storm DR, Xia Z. Pan YW, et al. J Biol Chem. 2012 Jul 6;287(28):23306-17. doi: 10.1074/jbc.M112.344762. Epub 2012 May 29. J Biol Chem. 2012. PMID: 22645146 Free PMC article. - Adult neurogenesis in the mammalian dentate gyrus.
Abbott LC, Nigussie F. Abbott LC, et al. Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review. - Social Cues, Adult Neurogenesis, and Reproductive Behavior.
Peretto P, Paredes RG. Peretto P, et al. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. PMID: 24830028 Free Books & Documents. Review.
Cited by
- Cellular junction dynamics and Alzheimer's disease: a comprehensive review.
Asghari K, Niknam Z, Mohammadpour-Asl S, Chodari L. Asghari K, et al. Mol Biol Rep. 2024 Feb 1;51(1):273. doi: 10.1007/s11033-024-09242-w. Mol Biol Rep. 2024. PMID: 38302794 Review. - SYK-623, a δ Opioid Receptor Inverse Agonist, Mitigates Chronic Stress-Induced Behavioral Abnormalities and Disrupted Neurogenesis.
Iwai T, Mishima R, Hirayama S, Nakajima H, Oyama M, Watanabe S, Fujii H, Tanabe M. Iwai T, et al. J Clin Med. 2024 Jan 21;13(2):608. doi: 10.3390/jcm13020608. J Clin Med. 2024. PMID: 38276114 Free PMC article. - Inducible and Conditional Activation of Adult Neurogenesis Rescues Cadmium-Induced Hippocampus-Dependent Memory Deficits in ApoE4-KI Mice.
Matsushita MT, Wang H, Abel GM, Xia Z. Matsushita MT, et al. Int J Mol Sci. 2023 May 23;24(11):9118. doi: 10.3390/ijms24119118. Int J Mol Sci. 2023. PMID: 37298071 Free PMC article. - Behavioral pattern separation is associated with neural and electrodermal correlates of context-dependent fear conditioning.
Neudert MK, Schäfer A, Zehtner RI, Fricke S, Seinsche RJ, Kruse O, Stark R, Hermann A. Neudert MK, et al. Sci Rep. 2023 Apr 5;13(1):5577. doi: 10.1038/s41598-023-31504-z. Sci Rep. 2023. PMID: 37019951 Free PMC article. - Morris Water Maze and Contextual Fear Conditioning Tasks to Evaluate Cognitive Functions Associated With Adult Hippocampal Neurogenesis.
Hernández-Mercado K, Zepeda A. Hernández-Mercado K, et al. Front Neurosci. 2022 Jan 3;15:782947. doi: 10.3389/fnins.2021.782947. eCollection 2021. Front Neurosci. 2022. PMID: 35046769 Free PMC article. Review.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous