M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination - PubMed (original) (raw)
. 2013 Sep;16(9):1211-1218.
doi: 10.1038/nn.3469. Epub 2013 Jul 21.
Affiliations
- PMID: 23872599
- PMCID: PMC3977045
- DOI: 10.1038/nn.3469
M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination
Veronique E Miron et al. Nat Neurosci. 2013 Sep.
Abstract
The lack of therapies for progressive multiple sclerosis highlights the need to understand the regenerative process of remyelination that can follow CNS demyelination. This involves an innate immune response consisting of microglia and macrophages, which can be polarized to distinct functional phenotypes: pro-inflammatory (M1) and anti-inflammatory or immunoregulatory (M2). We found that a switch from an M1- to an M2-dominant response occurred in microglia and peripherally derived macrophages as remyelination started. Oligodendrocyte differentiation was enhanced in vitro with M2 cell conditioned media and impaired in vivo following intra-lesional M2 cell depletion. M2 cell densities were increased in lesions of aged mice in which remyelination was enhanced by parabiotic coupling to a younger mouse and in multiple sclerosis lesions that normally show remyelination. Blocking M2 cell-derived activin-A inhibited oligodendrocyte differentiation during remyelination in cerebellar slice cultures. Thus, our results indicate that M2 cell polarization is essential for efficient remyelination and identify activin-A as a therapeutic target for CNS regeneration.
Figures
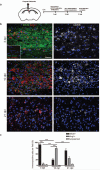
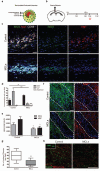
Figure 1. A switch from an M1 to an M2-dominant microglia/macrophage response occurs at the initiation of remyelination.
(a) Oligodendroglial lineage cell responses in the corpus callosum at 3, 10, and 21 days post lesion (dpl) induction by stereotactic injection of lysolecithin (LPC). (b) Lesions immunostained against iNOS (green), Arg-1 (red), and CD68 (white) at 3, 10 and 21 dpl. Inset: isotype controls. Scale bar, 25 μm. (c) Percentage of iNOS+ M1 cells, Arg-1+ M2 cells, and iNOS-Arg-1-(unpolarized) cells per field in lesion ± s.e.m.. One way ANOVA and Newman-Keuls post-hoc test, ***p<0.001 (_n_=5 mice, df=44).
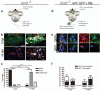
Figure 2. Microglia and peripherally-derived macrophages contribute to both M1 and M2 polarized populations during remyelination.
(a) _Ccr2_−/− mice were lesioned in the corpus callosum to examine polarization in microglia only. (b) _Ccr2_−/− lesions at 3 and 10 dpl immunostained against iNOS (green), Arg-1 (red) and CD68 (white). Scale bar, 25 μm. (c) Percentage of iNOS+, Arg-1+, or unpolarized (iNOS-, Arg-1-) cells in _Ccr2_−/− lesions ± s.e.m.. One way ANOVA and Newman-Keuls post-hoc test, ***p<0.001 (_n_=2-3 mice, df=14). (d) _Ccr2_−/−mice were lesioned in the corpus callosum and injected with GFP-expressing wildtype bone marrow derived cells to examine polarization in peripherally-derived macrophages only. (e) GFP+ macrophages (stars) and GFP-microglia (hash symbols) were iNOS+ (white) or Arg-1+ (red). Scale bar, 5 μm. (f) Numbers of GFP+ (macrophages) (black bars) and GFP-(microglia)(white bars) iNOS+ M1 and Arg-1+ M2 cells per field ± s.e.m. (_n_=3 mice).
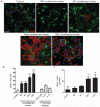
Figure 3. M2 conditioned media promotes oligodendrocyte differentiation.
(a) OPCs treated with unconditioned (control) or M1, M2a, or M2c conditioned media immunostained against NG2 (green) and MBP (red). Scale bar, 50 μm. (b) Percentage of MBP+ cells ± s.e.m. following treatment with microglia conditioned media, or polarizing factors alone as a control., Kruskal-Wallis test and Dunn’s multiple comparison post-test, ***p<0.001 (_n_=3 separate experiments). (c) Number of MOG+ cells per field ± s.e.m. following treatment with microglia conditioned media. Kruskal-Wallis test and Dunn’s multiple comparison post-test, **p<0.01 (_n_=3 separate experiments).
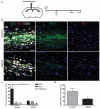
Figure 4. Selective depletion of M1 microglia/macrophages in a demyelinated lesion in the CNS impairs OPC proliferation.
(a) Gadolinium chloride (GdCl3) was injected into corpus callosum lesions at the onset of demyelination (0 dpl) prior to the peak in M1 polarization at 3 dpl. (b) Representative images of control or GdCl3-injected lesions at 3 dpl with immunostaining against iNOS (green), Arg-1 (red), and CD68 (white). Scale bar, 25 μm. (c) Numbers of iNOS+ M1, Arg-1+ M2, or unpolarized (iNOS-, Arg-1-) cells per field ± s.e.m. in control and GdCl3-injected lesions at 3 dpl. One way ANOVA and Newman-Keuls post-test, **p<0.01 (_n_=5 mice, df=29). (d) Density of PCNA+ Nkx2.2+ proliferating OPCs per mm2 in control and GdCl3-injected lesions ± s.e.m. at 3 dpl. _P_=0.0496, 2-tailed Student’s _t_-test (_n_=5 mice, df=8).
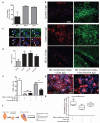
Figure 5. Selective depletion of M2 microglia/macrophages in a demyelinated lesion in the CNS impairs oligodendrocyte differentiation.
(a) Mannosylated clodronate liposomes were used to induce apoptosis selectively in M2 polarized microglia/macrophages due to upregulation of mannose receptor, and induce clodronate-mediated apoptosis following phagocytosis. (b) Corpus callosum lesions were injected with MCLs at 8 dpl prior to the peak in M2 polarization at 10 dpl. (c) Representative images at 10 dpl of control and MCL-injected lesions immunostained for iNOS (green), Arg-1 (red), and CD68 (white). Scale bar, 25 μm. (d) Number of iNOS+ M1, Arg-1+ M2, or unpolarized (iNOS-, Arg-1-) cells per field ± s.e.m. in control and MCL-injected lesions at 10 dpl. One way ANOVA and Newman-Keuls post-test, **p<0.01, ***p<0.001 (_n_=5 mice, df=29). (e) Mean lesion pixel counts ± s.e.m. for MAG and MBP at 10 dpl. 2-tailed Student’s _t_-test versus control, _P_=0.0361 and 0.0106, respectively (_n_=4-5 mice, df=7). (f) Representative images of lesions (dotted outline) at 10 dpl in control and MCL-injected lesions immunostained against MBP (red) and MAG (green). Scale bar, 100 μm. (g) Quantification of nodes of Ranvier (paranodal Caspr expression flanking nodal Ankyrin-G expression) ± s.e.m. in control and MCL-treated lesions at 21 dpl. 2-tailed Student’s _t_-test versus control, _P_=0.0068 (_n_=6 mice, df=10). Box plots indicate median (centre line), 25th -75th percentile (box limits) and minimum and maximum (whiskers). (h) Representative images of control and MCL-treated lesions at 21 dpl immunostained against Caspr (green) and Ankyrin-G (red). Scale bar, 25 μm.
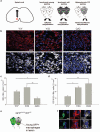
Figure 6. Restored remyelination efficiency in aged mice via heterochronic parabiosis is associated with increased densities of M2 polarized cells.
(a) Lesion induction in the ventral spinal cord by lysolecithin injection. Parabiotic pairings between young (Y-Y) (white) or old (O-O) (dark gray) animals were compared to lesions in old animals following heterochronic pairing (Y-O) (light gray), with previous studies showing restored remyelination efficiency in old partners of Y-O pairings. (b) Lesions immunostained at 7 dpl against Arg-1 (red) and CD68 (white). Scale bar, 25 μm. (c) MR+ IB4+ M2 cells/0.1 mm2 ± s.e.m. at 7 dpl. 2-tailed Student’s _t_-test: Y/Y vs. O/O, _P_=0.048; Y/O vs. O/O, _P_=0.005 (_n_=4 (O/O), 6(Y/O), 4 (Y/Y), df=13). (d) CD16/32+ IB4+ cells/0.1 mm2 ± s.e.m. at 7 dpl. 2-tailed Student’s _t_-test: Y/Y vs. O/O, _P_=0.0027; Y/Y vs. Y/O, _P_=0.014 (_n_=4 (O/O), 6(Y/O), 4 (Y/Y), df=13). (e) Parabiosis between a GFP-expressing young mouse and wildtype (WT) old mouse with a spinal cord lesion. (f) GFP expressing iNOS+ CD68+ M1 macrophages and Arg-1+ CD68+ M2 macrophages. Scale bar, 5 μm.
Figure 7. M2 microglia/macrophage densities are increased in acute active and the rim of chronic active multiple sclerosis lesions.
(a) Total CD68+ microglia/macrophages/mm2 were significantly increased in acute active (_P_=0.001), chronic active rim (_P_=0.0156) and centre (_P_=0.0156), chronic inactive (_P_=0.001) and remyelinated lesions (_P_=0.002). (b) iNOS+ M1 microglia/macrophages/mm2 were increased in acute active (_P_=0.001), chronic active rim (_P_=0.0156) and centre (_P_=0.0313), chronic inactive (_P_=0.0005), and remyelinated lesions (P<0.0001). (c) MR+ M2 microglia/macrophages/mm2 were increased in acute active lesions (_P_=0.0068) and the rim of chronic active lesions (_P_=0.0156). Mann-Whitney test. n for each lesion type indicated in Supplementary Table 1 online. (d) Representative image of MS lesion with iNOS immunolabeling. Scale bar, 100 μm. (e) Representative image of MS lesion with MR immunolabeling. Scale bar, 100 μm. (f) Co-localization of MR (top) and iNOS (bottom) with microglia/macrophage marker CD68 (arrowheads). Scale bar, 25 μm. (g) MR+ CD68+ M2 cells (arrows) were sometimes associated with blood vessels. Scale bar, 25 μm.
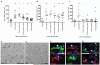
Figure 8. Activin-A is an M2-derived factor that drives oligodendrocyte differentiation during remyelination.
(a) Percentage of activin-A immunopositive cells per field in M1, M2a, and M2c microglia ± s.e.m. (b) Control corpus callosum lesions at 3 and 10 dpl (top and middle panels) and MCL-injected lesion at 10 dpl (bottom panel) immunostained for activin-A (red) and iNOS or MR (green). Scale bar, 25 μm. (c) Representative images of NG2+ OPCs (green) in remyelinating lesions expressing activin receptors Acvr2A, Acvr2B, and Acvr1B (red; arrows). Scale bar, 10 μm. (d) Percentage of MBP+ cells ± s.e.m. in OPC cultures treated with activin-A. One way ANOVA and Newman-Keuls post-hoc test, *p<0.05 (_n_=4 separate experiments, df=15). (e) Percentage of MBP+ cells ± s.e.m. in OPC cultures treated with combinations of M2a conditioned media, goat isotype control IgG, or goat anti-activin-A antibody. One way ANOVA and Newman-Keuls post-hoc test, **p<0.01 (_n_=4 separate experiments, df=11), ns=p>0.05. (f) 300 μm sagittal sections of cerebellum and attached hindbrain were taken from newborn mouse brain to obtain organotypic cultures, demyelinated with lysolecithin (LPC) and treated with M2 conditioned media and either anti-activin-A IgG or control goat IgG. (g) Representative images of slices treated with M2 conditioned media and goat IgG (left) or anti-activin-A antibody (right) immunostained for CC1 (white) and MBP (red). Scale bar, 25 μm. (h) Percentage of CC1+ oligodendrocytes normalized to values obtained from slices treated with IgG alone. 2-tailed one-sample or Student’s _t_-test (respectively), M2 conditioned media + IgG vs. IgG, _P_=0.0213; M2 conditioned media + IgG vs. M2 conditioned media + anti-activin-A IgG, _P_=0.0071 (_n_=8 slices, df=14). Box plots indicate median (centre line), 25th-75th percentile (box limits), minimum and maximum (whiskers).
Comment in
- Multiple sclerosis: Regenerative therapies for MS-hope on the horizon.
Malpass K. Malpass K. Nat Rev Neurol. 2013 Sep;9(9):484. doi: 10.1038/nrneurol.2013.158. Epub 2013 Aug 13. Nat Rev Neurol. 2013. PMID: 23938741 No abstract available.
Similar articles
- Oligodendrocyte Regeneration and CNS Remyelination Require TACE/ADAM17.
Palazuelos J, Klingener M, Raines EW, Crawford HC, Aguirre A. Palazuelos J, et al. J Neurosci. 2015 Sep 2;35(35):12241-7. doi: 10.1523/JNEUROSCI.3937-14.2015. J Neurosci. 2015. PMID: 26338334 Free PMC article. - Retinoid X receptor gamma signaling accelerates CNS remyelination.
Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van Evercooren A, Chambon P, Ffrench-Constant C, Franklin RJM. Huang JK, et al. Nat Neurosci. 2011 Jan;14(1):45-53. doi: 10.1038/nn.2702. Epub 2010 Dec 5. Nat Neurosci. 2011. PMID: 21131950 Free PMC article. - Fractalkine enhances oligodendrocyte regeneration and remyelination in a demyelination mouse model.
de Almeida MMA, Watson AES, Bibi S, Dittmann NL, Goodkey K, Sharafodinzadeh P, Galleguillos D, Nakhaei-Nejad M, Kosaraju J, Steinberg N, Wang BS, Footz T, Giuliani F, Wang J, Sipione S, Edgar JM, Voronova A. de Almeida MMA, et al. Stem Cell Reports. 2023 Feb 14;18(2):519-533. doi: 10.1016/j.stemcr.2022.12.001. Epub 2023 Jan 5. Stem Cell Reports. 2023. PMID: 36608690 Free PMC article. - Macrophages and CNS remyelination.
Miron VE, Franklin RJ. Miron VE, et al. J Neurochem. 2014 Jul;130(2):165-71. doi: 10.1111/jnc.12705. Epub 2014 Mar 26. J Neurochem. 2014. PMID: 24601941 Review. - The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination.
Keirstead HS, Blakemore WF. Keirstead HS, et al. Adv Exp Med Biol. 1999;468:183-97. doi: 10.1007/978-1-4615-4685-6_15. Adv Exp Med Biol. 1999. PMID: 10635029 Review.
Cited by
- Overview of emerging therapies for demyelinating diseases.
Medina R, Derias AM, Lakdawala M, Speakman S, Lucke-Wold B. Medina R, et al. World J Clin Cases. 2024 Oct 26;12(30):6361-6373. doi: 10.12998/wjcc.v12.i30.6361. World J Clin Cases. 2024. PMID: 39464332 Free PMC article. Review. - [Mechanisms underlying remyelination with special focus on demyelination models of multiple sclerosis].
Zheng S, Zhao J. Zheng S, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 Aug 25;49(4):524-530. doi: 10.3785/j.issn.1008-9292.2020.08.12. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 32985167 Free PMC article. Review. Chinese. - The impact of high and low dose ionising radiation on the central nervous system.
Betlazar C, Middleton RJ, Banati RB, Liu GJ. Betlazar C, et al. Redox Biol. 2016 Oct;9:144-156. doi: 10.1016/j.redox.2016.08.002. Epub 2016 Aug 10. Redox Biol. 2016. PMID: 27544883 Free PMC article. Review. - Repetitive Mild Traumatic Brain Injury in the Developing Brain: Effects on Long-Term Functional Outcome and Neuropathology.
Fidan E, Lewis J, Kline AE, Garman RH, Alexander H, Cheng JP, Bondi CO, Clark RS, Dezfulian C, Kochanek PM, Kagan VE, Bayır H. Fidan E, et al. J Neurotrauma. 2016 Apr 1;33(7):641-51. doi: 10.1089/neu.2015.3958. Epub 2015 Dec 1. J Neurotrauma. 2016. PMID: 26214116 Free PMC article. - Activin Signaling in the Pathogenesis and Therapy of Neuropsychiatric Diseases.
Link AS, Zheng F, Alzheimer C. Link AS, et al. Front Mol Neurosci. 2016 May 10;9:32. doi: 10.3389/fnmol.2016.00032. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27242425 Free PMC article. Review.
References
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. - PubMed
- Cash E, Zhang Y, Rott O. Microglia present myelin antigens to T cells after phagocytosis of oligodendrocytes. Cell Immunol. 1993;147:129–138. - PubMed
- Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol. Dis. 2005;18:166–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- 089000/WT_/Wellcome Trust/United Kingdom
- G0802545/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 079249/WT_/Wellcome Trust/United Kingdom
- G0300336/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous