Microbial reprogramming inhibits Western diet-associated obesity - PubMed (original) (raw)
. 2013 Jul 10;8(7):e68596.
doi: 10.1371/journal.pone.0068596. Print 2013.
Markus Kleinewietfeld, Christopher Smillie, Tatiana Levkovich, Alison Perrotta, Siddheshvar Bhela, Bernard J Varian, Yassin M Ibrahim, Jessica R Lakritz, Sean M Kearney, Antonis Chatzigiagkos, David A Hafler, Eric J Alm, Susan E Erdman
Affiliations
- PMID: 23874682
- PMCID: PMC3707834
- DOI: 10.1371/journal.pone.0068596
Microbial reprogramming inhibits Western diet-associated obesity
Theofilos Poutahidis et al. PLoS One. 2013.
Abstract
A recent epidemiological study showed that eating 'fast food' items such as potato chips increased likelihood of obesity, whereas eating yogurt prevented age-associated weight gain in humans. It was demonstrated previously in animal models of obesity that the immune system plays a critical role in this process. Here we examined human subjects and mouse models consuming Westernized 'fast food' diet, and found CD4(+) T helper (Th)17-biased immunity and changes in microbial communities and abdominal fat with obesity after eating the Western chow. In striking contrast, eating probiotic yogurt together with Western chow inhibited age-associated weight gain. We went on to test whether a bacteria found in yogurt may serve to lessen fat pathology by using purified Lactobacillus reuteri ATCC 6475 in drinking water. Surprisingly, we discovered that oral L. reuteri therapy alone was sufficient to change the pro-inflammatory immune cell profile and prevent abdominal fat pathology and age-associated weight gain in mice regardless of their baseline diet. These beneficial microbe effects were transferable into naïve recipient animals by purified CD4(+) T cells alone. Specifically, bacterial effects depended upon active immune tolerance by induction of Foxp3(+) regulatory T cells (Treg) and interleukin (Il)-10, without significantly changing the gut microbial ecology or reducing ad libitum caloric intake. Our finding that microbial targeting restored CD4(+) T cell balance and yielded significantly leaner animals regardless of their dietary 'fast food' indiscretions suggests population-based approaches for weight management and enhancing public health in industrialized societies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
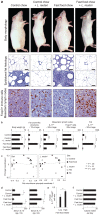
Figure 1. Eating probiotics blocks a gut microbiota-immunity-obesity axis.
L. reuteri protects mice from Western diet-associated obesity. Data are shown in male outbred Swiss mice at the age of 5 months. Numerous crown-like structures (CLS) caused by adipocyte death-related inflammation, and focal pyogranulomatous inflammation (PGI) arise in abdominal fat of ‘fast food’-fed but not probiotic-fed animals. Probiotics increase anti-inflammatory Foxp3+ regulatory (Treg) cells and reduce pro-inflammatory Il17 protein to restore immune balance coinciding with a slender physique (a and b), without restructuring GI microbial communities (c). In the same mice, serum cytokine analysis shows that the pro-inflammatory Il-17-associated effect of obesity is systemic, and that L. reuteri negates this effect up-regulating the anti-inflammatory cytokine Il-10 (d). Humans frequently eating ‘fast food’ also show an elevated ratio of pro-inflammatory IL17+/anti-inflammatory Foxp3+ Treg in peripheral blood cells compared to subjects never eating ‘fast food’ (e). Probiotic-consuming slim mice chose similar calories when compared with obese animals, regardless of baseline diet, highlighting potential for translational medicine (f). Fat histology: Hematoxylin and eosin, Bars = 50 µm; MLN Immunohistochemistry: Diaminobenzidine chromogen, hematoxylin counterstain, Bars = 8.3 µm.
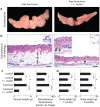
Figure 2. Dietary supplementation with L. reuteri protects from age-associated Western diet obesity.
Specifically, the abdominal (epididymal) fat mass is significantly reduced in probiotic-consuming Swiss mice (a). The slenderizing effect of L. reuteri is also observed in the subcutaneous fat depot. The subcutaneous fat layer (SF) is significantly thicker and has many CLS (inset) in “fast food”-fed mice in contrast to mice eating the same diet and L. reuteri. There is thicker dermis and increased subcutaneous hair follicle profiles in the left inset of the “fast food”+probiotic skin image (b). Fad pad weight and subcutaneous fat thickness histomorphometric analyses show that probiotics protect from age-associated obesity irrespective of baseline diet (c). Eating probiotics benefits aged Swiss mice as well as the young animals, evident here from the body weight analysis of 7- and 9-months-old male and female mice (d). Skin histology: Hematoxylin and eosin, Bars = 250 µm.
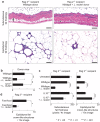
Figure 3. Mice exhibit an Interleukin 10-dependent Treg cell-mediated gut microbiota-immunity-obesity axis.
Genetically-inbred C57BL/6 strain mice similarly benefit from probiotic protection against Western diet-associated obesity and fat pathology, including CLS and focal pyogranulomatous inflammation as shown here in males at 5 months of age (a). _Interleukin (Il) 10_-deficient C57BL/6 male mice eating Westernized chow failed to benefit from oral L. reuteri supplementation. Mice from both experimental groups were obese and had increased CLS that were often seen coalescing to form focally diffuse areas of adipocyte necrosis (b). Fat histology: Hematoxylin and eosin. Bars = 250 µm (a) and 100 µm (b).
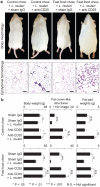
Figure 4. Diet-associated fat pathology is transferable to naïve animals using purified T-lymphocytes.
Adoptive transfer of purified CD4+ cells from C57BL/6 wild type donor mice eating probiotics into C57BL/6 _Rag2_-null mice was sufficient to significantly reduce recipient body fat depots such as subcutaneous fat, as well as ameliorate abdominal fat pathology (a). Control diet-fed mice used as donors for lymphocyte transfer experiments showed IL10-dependent L.reuteri benefits including significantly less CLS in their abdominal fat (b). This immune-mediated protection requires Il10, as adoptive transfer of CD4+ cells from probiotic-fed Il10-deficient donors did not protect the recipient mice from obesity and associated fat pathology (c). Purified wild type CD4+ FoxP3+ Treg cells from mice eating L. reuteri were sufficient for beneficial effects in _Rag2_-null recipient mice rescuing them from obesity-associated pathology (d). Skin and fat histology: Hematoxylin and eosin. Bars skin = 250 µm, edididymal fat = 50 µm.
Figure 5. Anti-obesity protection of oral probiotics in outbred Swiss mice requires CD25+ immune cells.
Depletion of CD4+CD25+ Treg cells entirely inhibits probiotic-induced protection from age-associated obesity and abdominal fat pathology (a). Probiotics protect from weight gain unless mice were simultaneously treated with anti-CD25 antibody, in which case animals rapidly became obese. Frequency of prototype crown-like structures was increased in abdominal fat after depletion of CD25+ cells but not in sham IgG-treated control animals (b). Fat histology: Hematoxylin and eosin. Bars = 100 µm.
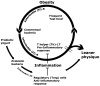
Figure 6. Proposed mechanistic overview.
In order to explain the ‘fast food’ versus ‘yogurt’ age-associated weight disparity observed in human subjects, we propose that Western-style dietary habits alter gut microbiota fueling an IL-6 driven, IL-17-dominant, systemic smoldering inflammatory milieu, ultimately leading to a vicious circle of obesity and inflammation. On the other hand, individuals consuming probiotic yogurt enrich their gut with probiotic bacteria that stimulate the anti-inflammatory arm of the immune system. Potent IL-10-associated Treg responses in these individuals rescue them from the inflammation-obesity cycle, thus increasing likelihood of a leaner physique.
Similar articles
- Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice.
Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Lakritz JR, et al. Int J Cancer. 2014 Aug 1;135(3):529-40. doi: 10.1002/ijc.28702. Epub 2014 Jan 10. Int J Cancer. 2014. PMID: 24382758 Free PMC article. - Secretion of Recombinant Interleukin-22 by Engineered Lactobacillus reuteri Reduces Fatty Liver Disease in a Mouse Model of Diet-Induced Obesity.
Oh JH, Schueler KL, Stapleton DS, Alexander LM, Yen CE, Keller MP, Attie AD, van Pijkeren JP. Oh JH, et al. mSphere. 2020 Jun 24;5(3):e00183-20. doi: 10.1128/mSphere.00183-20. mSphere. 2020. PMID: 32581074 Free PMC article. - Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity.
Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Park DY, et al. PLoS One. 2013;8(3):e59470. doi: 10.1371/journal.pone.0059470. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555678 Free PMC article. - Microbiota and Probiotics: The Role of Limosilactobacillus Reuteri in Diverticulitis.
Piccioni A, Franza L, Vaccaro V, Saviano A, Zanza C, Candelli M, Covino M, Franceschi F, Ojetti V. Piccioni A, et al. Medicina (Kaunas). 2021 Aug 5;57(8):802. doi: 10.3390/medicina57080802. Medicina (Kaunas). 2021. PMID: 34441008 Free PMC article. Review. - The Potential Role of Yogurt in Weight Management and Prevention of Type 2 Diabetes.
Panahi S, Tremblay A. Panahi S, et al. J Am Coll Nutr. 2016 Nov-Dec;35(8):717-731. doi: 10.1080/07315724.2015.1102103. Epub 2016 Jun 22. J Am Coll Nutr. 2016. PMID: 27332081 Review.
Cited by
- Lactobacillus reuteri in Its Biofilm State Improves Protection from Experimental Necrotizing Enterocolitis.
Al-Hadidi A, Navarro J, Goodman SD, Bailey MT, Besner GE. Al-Hadidi A, et al. Nutrients. 2021 Mar 12;13(3):918. doi: 10.3390/nu13030918. Nutrients. 2021. PMID: 33809097 Free PMC article. Review. - Effects of Two Distinct Psychoactive Microbes, Lacticaseibacillus rhamnosus JB-1 and Limosilactobacillus reuteri 6475, on Circulating and Hippocampal mRNA in Male Mice.
Haas-Neill S, Iwashita E, Dvorkin-Gheva A, Forsythe P. Haas-Neill S, et al. Int J Mol Sci. 2022 Aug 25;23(17):9653. doi: 10.3390/ijms23179653. Int J Mol Sci. 2022. PMID: 36077051 Free PMC article. - Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice.
Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, Kim DD, Kim HS. Lee J, et al. BMC Microbiol. 2016 Apr 19;16:69. doi: 10.1186/s12866-016-0686-7. BMC Microbiol. 2016. PMID: 27095067 Free PMC article. - Consuming cholera toxin counteracts age-associated obesity.
Varian BJ, Poutahidis T, Haner G, Hardas A, Lau V, Erdman SE. Varian BJ, et al. Oncotarget. 2019 Sep 17;10(53):5497-5509. doi: 10.18632/oncotarget.27137. eCollection 2019 Sep 17. Oncotarget. 2019. PMID: 31565184 Free PMC article. - Long-Term Potable Effects of Alkalescent Mineral Water on Intestinal Microbiota Shift and Physical Conditioning.
Yahiro T, Hara T, Matsumoto T, Ikebe E, Fife-Koshinomi N, Xu Z, Hiratsuka T, Iha H, Inomata M. Yahiro T, et al. Evid Based Complement Alternat Med. 2019 Nov 19;2019:2710587. doi: 10.1155/2019/2710587. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31827547 Free PMC article.
References
- Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169–2180. - PubMed
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, et al. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46: 2347–2355. - PubMed
- West M (2009) Dead adipocytes and metabolic dysfunction: recent progress. Curr Opin Endocrinol Diabetes Obes 16: 178–182. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA164337/CA/NCI NIH HHS/United States
- U19 AI070352/AI/NIAID NIH HHS/United States
- R01 AI091568/AI/NIAID NIH HHS/United States
- P01 AI039671/AI/NIAID NIH HHS/United States
- R01 CA108854/CA/NCI NIH HHS/United States
- P01 AI045757/AI/NIAID NIH HHS/United States
- R01CA108854/CA/NCI NIH HHS/United States
- U19 AI046130/AI/NIAID NIH HHS/United States
- P01AI045757/AI/NIAID NIH HHS/United States
- P30-ES002109/ES/NIEHS NIH HHS/United States
- T32 GM087237/GM/NIGMS NIH HHS/United States
- P30 ES002109/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials