Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease - PubMed (original) (raw)
Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease
Laura R Marks et al. mBio. 2013.
Abstract
Streptococcus pneumoniae is a common human nasopharyngeal commensal colonizing 10% to 40% of healthy individuals, depending on age. Despite a low invasive disease rate, widespread carriage ensures that infection occurs often enough to make S. pneumoniae a leading bacterial cause of respiratory disease worldwide. However, the mechanisms behind transition from asymptomatic colonization to dissemination and disease in otherwise sterile sites remain poorly understood but are epidemiologically strongly linked to infection with respiratory viruses. In this report, we show that infection with influenza A virus and treatment with the resulting host signals (febrile-range temperatures, norepinephrine, extracytoplasmic ATP, and increased nutrient availability) induce the release of bacteria from biofilms in a newly developed biofilm model on live epithelial cells both in vitro and during in vivo colonization. These dispersed bacteria have distinct phenotypic properties different from those of both biofilm and broth-grown, planktonic bacteria, with the dispersed population showing differential virulence gene expression characteristics resulting in a significantly increased ability to disseminate and cause infection of otherwise sterile sites, such as the middle ear, lungs, and bloodstream. The results offer novel and important insights into the role of interkingdom signaling between microbe and host during biofilm dispersion and transition to acute disease.
Importance: This report addresses the mechanisms involved in transition from pneumococcal asymptomatic colonization to disease. In this study, we determined that changes in the nasopharyngeal environment result in the release of bacteria from colonizing biofilms with a gene expression and virulence phenotype different not only from that of colonizing biofilm bacteria but also from that of the broth-grown planktonic bacteria commonly used for pathogenesis studies. The work importantly also identifies specific host factors responsible for the release of bacteria and their changed phenotype. We show that these interkingdom signals are recognized by bacteria and are induced by influenza virus infection, which is epidemiologically strongly associated with transition to secondary pneumococcal disease. As virus infection is a common inducer of transition to disease among species occupying the nasopharynx, the results of this study may provide a basis for better understanding of the signals involved in the transition from colonization to disease in the human nasopharynx.
Figures
FIG 1
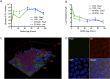
Establishment of a pneumococcal biofilm model on live epithelial cells. S. pneumoniae D39 or EF3030 bacteria were seeded on paraformaldehyde-fixed HRECs. At 48 h, biofilms were transplanted to live HRECs. (A and B) Biofilm biomass (A) and sensitivity to gentamicin (500 µg/ml) (B) were measured by viable counts over time. Green lines indicate bacterial biofilms on fixed epithelia, and blue lines represent the bacteria after transplantation to live epithelial cells. (C) Representative three-dimensional (3D) reconstruction of confocal image of a D39 biofilm at 48 h posttransplantation onto live HREC layers. Bacteria are stained red using Baclight Red, and epithelia were stained with 488-conjugated concanavalin A (green) and their nuclei with DAPI (blue). The numbers represent distances in micrometers. (D) Representative image from the midcell confocal section view of a D39 biofilm sample after 48 h on live HRECs showing no bacterial invasion. HRECs were stained with anti-secretory component (SC) antibody (red), nuclei were stained with DAPI (blue), and pneumococci were stained with anti-PspA antibody (green).
FIG 2
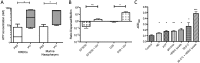
Dispersion of biofilm bacteria by virus infection and host factors. (A) The ATP concentrations in the supernatant of HRECs infected with influenza A virus (IAV) or in the nasopharyngeal lavage fluid recovered from mice 24 h after IAV infection were determined. (B) The impact of IAV infection on the ratio of bacteria in the supernatant (dispersed) to epithelium-associated bacteria (biofilm) 48 h after biofilm transplant to live HRECs was measured. (C) The change in the optical density at 600 nm (ΔOD600) of biofilm supernatant was measured 2 h after application of the indicated stimuli (NE = norepinephrine). The change in optical density correlated well with the number of CFU obtained from bacterial growth of the same supernatants. For each analysis, three independent assays were performed in duplicate. Statistical analysis was performed using Student’s t test (* = P < 0.05, ** = P < 0.01, *** = P < 0.001).
FIG 3
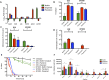
Characterization of pneumococcal populations for gene regulation, adherence, invasion, toxicity, and cytokine induction from exposed HRECs. Biofilm and dispersed and planktonic pneumococcal populations show distinct gene expression profiles of key virulence genes (P < 0.05 for all comparisons using 2-way analysis of variance [ANOVA]) (A) and differences in transparent- and opaque-phase states (*** = P < 0.001 using 1-way ANOVA). (C to F) The populations further differed in their ability to adhere to (C), invade (D), and kill (E) HRECs (** = P < 0.01 for all comparisons using 1-way ANOVA) and induce different levels of key cytokines involved in proinflammatory responses from the exposed HRECs (* = P < 0.05 using 2-way ANOVA) (F). Each experiment was repeated at least three times in duplicate. ns, nonsignificant.
FIG 4
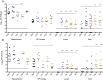
Colonization and dissemination of pneumococcal populations after intranasal inoculation, aspiration inoculation, or intraperitoneal inoculation. (A and B) Individual 6-week-old female BALB/cByJ mice were inoculated with biofilm (designated “B”) or dispersed (designated “D”) or broth-grown, planktonic (designated “P”) populations of EF3030 (A) and D39 (B) pneumococci intranasally without anesthesia to determine nasopharyngeal colonization and dissemination from the nasopharynx to the lungs and middle ears. Additionally, the bacterial burden in nasopharyngeal (NP) lavage fluid was determined. (C and D) Mice were furthermore inoculated with EF3030 (C) and D39 (D) pneumococci intranasally after anesthesia in an aspiration pneumonia model, and the bacterial burden in the lung tissue and dissemination to the bloodstream were determined by plate counts. (E and F) Finally, each bacterial population of EF3030 (E) and D39 (F) pneumococci was injected intraperitoneally to determine the respective levels of virulence upon reaching the vascular compartment. For both nonanesthetized and anesthetized intranasal challenges, nasal lavage fluids and all tissues were collected at 48 h postinfection. For intraperitoneal challenge, samples were collected at 24 h postinfection. Each dot in the graphs represents an individual mouse. An “X” represents a mouse that became moribund and required euthanasia before the end of the experiment. Each experiment included at least 6 mice. Statistical analysis was performed using one-way ANOVA (* = P < 0.05, ** = P < 0.01, and *** = P < 0.001, indicating significant differences between the populations).
FIG 5
Histological representation of mouse tissues infected with various pneumococcal populations. Representative histological images of nasal epithelium, middle ear space, and lungs 7 days following nonanesthetized intranasal challenge (rows 1 to 3) with PBS (mock-infected control), biofilm, or dispersed or planktonic populations. Row 4 shows histological sections of the lungs 48 h following intratracheal aspiration of these same bacterial populations. Tissues were stained with hematoxylin and eosin and examined microscopically at magnifications of ×400 for row 1 and ×200 for rows 2 to 4. The sections are labeled with relevant structures (LI, leukocyte infiltrate; TM, tympanic membrane; SM, stapes muscle; ME, middle ear cavity; Spn, S. pneumoniae).
FIG 6
Bacterial dissemination of mice stably colonized with pneumococci and challenged with influenza A virus. Bacterial burden in tissues and nasopharyngeal lavage fluid was measured for individual 6-week-old female BALB/cByJ mice colonized intranasally without anesthesia with EF3030 (A and C) or D39 (B and D) biofilm bacteria at 1 (A and B) or 5 (C and D) days postinfection with IAV. Each dot represents an individual mouse. Statistical analysis was performed using Student’s t test. * = P < 0.05, ** = P < 0.01, *** = P < 0.001.
FIG 7
Bacterial dissemination of mice stably colonized with pneumococci and challenged with dispersants. The bacterial burden in tissues and nasopharyngeal (NP) lavage fluid was measured for individual 6-week-old female BALB/cByJ mice colonized intranasally without anesthesia with biofilm bacteria from (A) EF3030 or (B) D39 pneumococci for 48 h and then challenged intranasally with 20 µl PBS, 10 mM ATP, 100 nM norepinephrine (NE), or 1 M glucose (Glu) or subjected to 4 h of febrile-range hyperthermia (FRH). Each experiment represents at least three individual experiments with duplicate samples. Statistical analysis was performed using Student’s t test, comparing treatment conditions to PBS control. * = P < 0.05, ** = P < 0.01, *** = P < 0.001.
Similar articles
- Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease.
Chao Y, Marks LR, Pettigrew MM, Hakansson AP. Chao Y, et al. Front Cell Infect Microbiol. 2015 Jan 13;4:194. doi: 10.3389/fcimb.2014.00194. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25629011 Free PMC article. Review. - Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection.
Pettigrew MM, Marks LR, Kong Y, Gent JF, Roche-Hakansson H, Hakansson AP. Pettigrew MM, et al. Infect Immun. 2014 Nov;82(11):4607-19. doi: 10.1128/IAI.02225-14. Epub 2014 Aug 18. Infect Immun. 2014. PMID: 25135685 Free PMC article. - High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae.
Marks LR, Reddinger RM, Hakansson AP. Marks LR, et al. mBio. 2012 Sep 25;3(5):e00200-12. doi: 10.1128/mBio.00200-12. Print 2012. mBio. 2012. PMID: 23015736 Free PMC article. - Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo.
Marks LR, Parameswaran GI, Hakansson AP. Marks LR, et al. Infect Immun. 2012 Aug;80(8):2744-60. doi: 10.1128/IAI.00488-12. Epub 2012 May 29. Infect Immun. 2012. PMID: 22645283 Free PMC article. - Pneumococci in biofilms are non-invasive: implications on nasopharyngeal colonization.
Gilley RP, Orihuela CJ. Gilley RP, et al. Front Cell Infect Microbiol. 2014 Nov 6;4:163. doi: 10.3389/fcimb.2014.00163. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25414838 Free PMC article. Review.
Cited by
- Type IV Pilus Expression Is Upregulated in Nontypeable Haemophilus influenzae Biofilms Formed at the Temperature of the Human Nasopharynx.
Mokrzan EM, Ward MO, Bakaletz LO. Mokrzan EM, et al. J Bacteriol. 2016 Sep 9;198(19):2619-30. doi: 10.1128/JB.01022-15. Print 2016 Oct 1. J Bacteriol. 2016. PMID: 27044626 Free PMC article. - The release of a distinct cell type from swarm colonies facilitates dissemination of Vibrio parahaemolyticus in the environment.
Freitas C, Glatter T, Ringgaard S. Freitas C, et al. ISME J. 2020 Jan;14(1):230-244. doi: 10.1038/s41396-019-0521-x. Epub 2019 Oct 17. ISME J. 2020. PMID: 31624347 Free PMC article. - Impact of viral upper respiratory tract infection on the concentration of nasopharyngeal pneumococcal carriage among Kenyan children.
Morpeth SC, Munywoki P, Hammitt LL, Bett A, Bottomley C, Onyango CO, Murdoch DR, Nokes DJ, Scott JAG. Morpeth SC, et al. Sci Rep. 2018 Jul 23;8(1):11030. doi: 10.1038/s41598-018-29119-w. Sci Rep. 2018. PMID: 30038420 Free PMC article. - Live attenuated influenza virus increases pneumococcal translocation and persistence within the middle ear.
Mina MJ, Klugman KP, Rosch JW, McCullers JA. Mina MJ, et al. J Infect Dis. 2015 Jul 15;212(2):195-201. doi: 10.1093/infdis/jiu804. Epub 2014 Dec 11. J Infect Dis. 2015. PMID: 25505300 Free PMC article. - Glutamate Dehydrogenase (GdhA) of Streptococcus pneumoniae Is Required for High Temperature Adaptation.
Gazioglu O, Kareem BO, Afzal M, Shafeeq S, Kuipers OP, Ulijasz AT, Andrew PW, Yesilkaya H. Gazioglu O, et al. Infect Immun. 2021 Nov 16;89(12):e0040021. doi: 10.1128/IAI.00400-21. Epub 2021 Sep 7. Infect Immun. 2021. PMID: 34491792 Free PMC article.
References
- Gray BM, Converse GM, III, Dillon HC. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923–933 - PubMed
- Millar EV, Watt JP, Bronsdon MA, Dallas J, Reid R, Santosham M, O’Brien KL. 2008. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin. Infect. Dis. 47:989–996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials