Effect of cilostazol on arterial stiffness and vascular adhesion molecules in type 2 diabetic patients with metabolic syndrome: a randomised, double-blind, placebo-controlled, crossover trial - PubMed (original) (raw)
Effect of cilostazol on arterial stiffness and vascular adhesion molecules in type 2 diabetic patients with metabolic syndrome: a randomised, double-blind, placebo-controlled, crossover trial
Nam Hoon Kim et al. Diabetol Metab Syndr. 2013.
Abstract
Background: The phosphodiesterase inhibitor cilostazol has beneficial effects on atherosclerosis by virtue of vasodilatory and antiplatelet effects. However, less is known about the effect of cilostazol on arterial stiffness and biochemical markers related to vascular inflammation and endothelial dysfunction in type 2 diabetic patients with metabolic syndrome.
Methods: In this randomized, double-blind, crossover trial, 45 diabetic patients with metabolic syndrome were randomly assigned to either the cilostazol group (50 mg for 2 weeks, 100 mg for 6 weeks) or placebo group for an 8-week treatment phase, and then crossed over. Brachial-ankle pulse wave velocity (baPWV) and serum levels of inflammatory cytokines and vascular cellular adhesion molecules were measured before and after each treatment phase.
Results: Compared with the placebo group, the mean baPWV did not improve in the cilostazol group (mean difference 31.42 cm/sec, 95% CI -55.67 to 118.5). Cilostazol treatment significantly reduced soluble vascular cellular adhesion molecule-1 (sVCAM-1) level (from 1288.7 ± 285.6 to 1168.2 ± 252.3 ng/dL, P = 0.0003), and there was also significant mean difference between groups (mean difference 105.18 ng/dL, 95% CI 10.65 to 199.71). However, other biochemical markers including lipid profiles, high sensitivity C-reactive protein, adiponectin, interleukin-6, tumor necrosis factor-alpha, monocyte chemotactic protein-1, and soluble intercellular adhesion molecule-1 did not improve with cilostazol treatment.
Conclusion: Cilostazol treatment significantly reduced serum sVCAM-1 level, but this short term treatment was not associated with beneficial effect on arterial stiffness and other inflammatory markers.
Trial registration: (Clinical trial reg. no. NCT00573950, clinicaltrials.gov.).
Figures
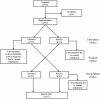
Figure 1
Trial design.
Similar articles
- The effects of cilostazol on exercise-induced ischaemia-reperfusion injury in patients with peripheral arterial disease.
O'Donnell ME, Badger SA, Sharif MA, Makar RR, McEneny J, Young IS, Lee B, Soong CV. O'Donnell ME, et al. Eur J Vasc Endovasc Surg. 2009 Mar;37(3):326-35. doi: 10.1016/j.ejvs.2008.11.028. Epub 2008 Dec 27. Eur J Vasc Endovasc Surg. 2009. PMID: 19112032 Clinical Trial. - Cilostazol effectively attenuates deterioration of albuminuria in patients with type 2 diabetes: a randomized, placebo-controlled trial.
Tang WH, Lin FH, Lee CH, Kuo FC, Hsieh CH, Hsiao FC, Hung YJ. Tang WH, et al. Endocrine. 2014 Mar;45(2):293-301. doi: 10.1007/s12020-013-0002-3. Epub 2013 Jun 18. Endocrine. 2014. PMID: 23775007 Clinical Trial. - Cilostazol attenuates the severity of peripheral arterial occlusive disease in patients with type 2 diabetes: the role of plasma soluble receptor for advanced glycation end-products.
Liu JS, Chuang TJ, Chen JH, Lee CH, Hsieh CH, Lin TK, Hsiao FC, Hung YJ. Liu JS, et al. Endocrine. 2015 Aug;49(3):703-10. doi: 10.1007/s12020-015-0545-6. Epub 2015 Feb 11. Endocrine. 2015. PMID: 25666934 Free PMC article. Clinical Trial. - Effect of cilostazol in treating diabetes-associated microvascular complications.
Asal NJ, Wojciak KA. Asal NJ, et al. Endocrine. 2017 May;56(2):240-244. doi: 10.1007/s12020-017-1279-4. Epub 2017 Mar 14. Endocrine. 2017. PMID: 28293857 Review. - Cilostazol for peripheral arterial disease.
Robless P, Mikhailidis DP, Stansby GP. Robless P, et al. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD003748. doi: 10.1002/14651858.CD003748.pub3. Cochrane Database Syst Rev. 2008. PMID: 18254032 Updated. Review.
Cited by
- Cilostazol for intermittent claudication.
Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Bedenis R, et al. Cochrane Database Syst Rev. 2014 Oct 31;2014(10):CD003748. doi: 10.1002/14651858.CD003748.pub4. Cochrane Database Syst Rev. 2014. PMID: 25358850 Free PMC article. Updated. Review. - Cilostazol for intermittent claudication.
Brown T, Forster RB, Cleanthis M, Mikhailidis DP, Stansby G, Stewart M. Brown T, et al. Cochrane Database Syst Rev. 2021 Jun 30;6(6):CD003748. doi: 10.1002/14651858.CD003748.pub5. Cochrane Database Syst Rev. 2021. PMID: 34192807 Free PMC article.
References
- Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24(3):278–301. - PubMed
- de Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn study. Arterioscler Thromb Vasc Biol. 2006;26(5):1086–1093. doi: 10.1161/01.ATV.0000215951.36219.a4. - DOI - PubMed
- Hayaishi-Okano R, Yamasaki Y, Katakami N, Ohtoshi K, Gorogawa S, Kuroda A, Matsuhisa M, Kosugi K, Nishikawa N, Kajimoto Y, Hori M. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care. 2002;25(8):1432–1438. doi: 10.2337/diacare.25.8.1432. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials