The placebo effect in clinical trials for alcohol dependence: an exploratory analysis of 51 naltrexone and acamprosate studies - PubMed (original) (raw)
Meta-Analysis
. 2013 Dec;37(12):2128-37.
doi: 10.1111/acer.12197. Epub 2013 Jul 24.
Affiliations
- PMID: 23889231
- PMCID: PMC3823636
- DOI: 10.1111/acer.12197
Meta-Analysis
The placebo effect in clinical trials for alcohol dependence: an exploratory analysis of 51 naltrexone and acamprosate studies
Raye Z Litten et al. Alcohol Clin Exp Res. 2013 Dec.
Abstract
Background: The placebo effect often undermines efforts to determine treatment effectiveness in clinical trials. A significant placebo response occurs in alcohol trials, but it is not well understood. The purpose of this study was to characterize the placebo response across multiple naltrexone and acamprosate studies.
Methods: Fifty-one trials, 3 with a naltrexone and an acamprosate arm, 31 with at least 1 naltrexone arm, and 17 with at least 1 acamprosate arm, were identified from Cochrane reviews and PubMed search. To be included in this study, patients had to be at least 18 years old, abstinent from alcohol before randomization, and meet a diagnosis of alcohol dependence. Pearson correlation coefficients (rp ) and simple linear regression were used to describe the strength of linear relationships between placebo response and treatment effect size. Spearman's rank correlation coefficients (rs ) were used to examine the strength of associations between study characteristics and placebo response.
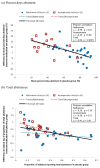
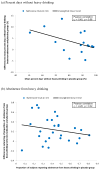
Results: For the end point measures of percent days abstinent and total abstinence, a negative relationship was evident between placebo response and treatment effect size in the naltrexone trials (rp = -0.55, p < 0.01 and rp = -0.20, p = 0.35, respectively) as well as in the acamprosate trials (rp = -0.45, p = 0.09 and rp = -0.56, p = 0.01, respectively). The placebo response for percent days abstinent was negatively correlated with mean age of participants (rs = -0.42, p = 0.05) across naltrexone trials and positively correlated with publication year (rs = 0.57, p = 0.03) across acamprosate trials. However, these 2 study characteristics were not significantly correlated with treatment effect size.
Conclusions: The placebo response varied considerably across trials and was negatively correlated with the treatment effect size. Additional studies are required to fully understand the complex nature of the placebo response and to evaluate approaches to minimize its effects.
Keywords: Acamprosate; Alcohol Dependence; Meta-Analysis; Naltrexone; Placebo Effect.
Copyright © 2013 by the Research Society on Alcoholism.
Figures
Figure 1
Relationships between placebo response (x–axis) and treatment effect size (y-axis) for endpoints (a) percent days abstinent and (b) total abstinence in naltrexone and acamprosate trials.
Figure 2
Relationships between placebo response (x-axis) and treatment effect size (y-axis) for endpoints (a) percent days without heavy drinking and (b) abstinence from heavy drinking in naltrexone trials.
Similar articles
- The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis.
Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. Donoghue K, et al. Addiction. 2015 Jun;110(6):920-30. doi: 10.1111/add.12875. Epub 2015 Mar 17. Addiction. 2015. PMID: 25664494 Review. - Study characteristics influence the efficacy of substance abuse treatments: A meta-analysis of medications for alcohol use disorder.
Klemperer EM, Hughes JR, Naud S. Klemperer EM, et al. Drug Alcohol Depend. 2018 Sep 1;190:229-234. doi: 10.1016/j.drugalcdep.2018.06.015. Epub 2018 Jul 24. Drug Alcohol Depend. 2018. PMID: 30059816 Review. - Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective.
Soyka M, Chick J. Soyka M, et al. Am J Addict. 2003;12(s1):s69-s80. doi: 10.1111/j.1521-0391.2003.tb00497.x. Am J Addict. 2003. PMID: 14972781 Review. - Gender differences in alcohol treatment: an analysis of outcome from the COMBINE study.
Greenfield SF, Pettinati HM, O'Malley S, Randall PK, Randall CL. Greenfield SF, et al. Alcohol Clin Exp Res. 2010 Oct;34(10):1803-12. doi: 10.1111/j.1530-0277.2010.01267.x. Epub 2010 Jul 20. Alcohol Clin Exp Res. 2010. PMID: 20645934 Free PMC article. - Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial.
Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A; COMBINE Study Research Group. Anton RF, et al. JAMA. 2006 May 3;295(17):2003-17. doi: 10.1001/jama.295.17.2003. JAMA. 2006. PMID: 16670409 Clinical Trial.
Cited by
- Consumption outcomes in clinical trials of alcohol use disorder treatment: Consideration of standard drink misestimation.
Kirouac M, Kruger E, Wilson AD, Hallgren KA, Witkiewitz K. Kirouac M, et al. Am J Drug Alcohol Abuse. 2019;45(5):451-459. doi: 10.1080/00952990.2019.1584202. Epub 2019 Mar 14. Am J Drug Alcohol Abuse. 2019. PMID: 30870054 Free PMC article. - Age and sex as moderators of the placebo response – an evaluation of systematic reviews and meta-analyses across medicine.
Weimer K, Colloca L, Enck P. Weimer K, et al. Gerontology. 2015;61(2):97-108. doi: 10.1159/000365248. Gerontology. 2015. PMID: 25427869 Free PMC article. Review. - Sodium Oxybate for Alcohol Dependence: A Network Meta-Regression Analysis Considering Population Severity at Baseline and Treatment Duration.
Guiraud J, Addolorato G, Aubin HJ, Bachelot S, Batel P, de Bejczy A, Benyamina A, Caputo F, Couderc M, Dematteis M, Goudriaan AE, Gual A, Lecoustey S, Lesch OM, Maremmani I, Nutt DJ, Paille F, Perney P, Rehm J, Rolland B, Scherrer B, Simon N, Söderpalm B, Somaini L, Sommer WH, Spanagel R, Walter H, van den Brink W. Guiraud J, et al. Alcohol Alcohol. 2023 Mar 10;58(2):125-133. doi: 10.1093/alcalc/agac070. Alcohol Alcohol. 2023. PMID: 36617267 Free PMC article. Review. - Study Characteristics Influence the Efficacy of Substance Abuse Treatments: A Meta-analysis of Medications for Smoking Cessation.
Klemperer EM, Hughes JR, Naud S. Klemperer EM, et al. Nicotine Tob Res. 2020 Mar 16;22(3):317-323. doi: 10.1093/ntr/nty225. Nicotine Tob Res. 2020. PMID: 30380134 Free PMC article. Review. - Acceptability and feasibility of a randomized clinical trial of oral naltrexone vs. placebo for women living with HIV infection: Study design challenges and pilot study results.
Cook RL, Weber KM, Mai D, Thoma K, Hu X, Brumback B, Karki M, Bryant K, Rathore M, Young M, Cohen M. Cook RL, et al. Contemp Clin Trials. 2017 Sep;60:72-77. doi: 10.1016/j.cct.2017.06.012. Epub 2017 Jun 19. Contemp Clin Trials. 2017. PMID: 28642209 Free PMC article. Clinical Trial.
References
- Ahmadi J, Ahmadi N. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol dependence. German J Psychiatry. 2002;5:85–89.
- American Psychiatric Association. Diagnotic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1980.
- American Psychiatric Association. Diagnotic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1987. revised.
- American Psychiatric Association. Diagnotic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington DC: 1994.
- Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, Thevos A, Wang W, Woolson R. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005;25:349–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical