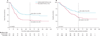Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial - PubMed (original) (raw)
Clinical Trial
. 2013 Sep;14(10):999-1008.
doi: 10.1016/S1470-2045(13)70309-7. Epub 2013 Jul 25.
Robert C Seeger, Katherine K Matthay, Wendy B London, Richard Sposto, Stephan A Grupp, Daphne A Haas-Kogan, Michael P Laquaglia, Alice L Yu, Lisa Diller, Allen Buxton, Julie R Park, Susan L Cohn, John M Maris, C Patrick Reynolds, Judith G Villablanca
Affiliations
- PMID: 23890779
- PMCID: PMC3963485
- DOI: 10.1016/S1470-2045(13)70309-7
Clinical Trial
Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial
Susan G Kreissman et al. Lancet Oncol. 2013 Sep.
Abstract
Background: Myeloablative chemoradiotherapy and immunomagnetically purged autologous bone marrow transplantation has been shown to improve outcome for patients with high-risk neuroblastoma. Currently, peripheral blood stem cells (PBSC) are infused after myeloablative therapy, but the effect of purging is unknown. We did a randomised study of tumour-selective PBSC purging in stem-cell transplantation for patients with high-risk neuroblastoma.
Methods: Between March 16, 2001, and Feb 24, 2006, children and young adults (<30 years) with high-risk neuroblastoma were randomly assigned at diagnosis by a web-based system (in a 1:1 ratio) to receive either non-purged or immunomagnetically purged PBSC. Randomisation was done in blocks stratified by International Neuroblastoma Staging System stage, age, MYCN status, and International Neuroblastoma Pathology classification. Patients and treating physicians were not masked to treatment assignment. All patients were treated with six cycles of induction chemotherapy, myeloablative consolidation, and radiation therapy to the primary tumour site plus meta-iodobenzylguanidine avid metastases present before myeloablative therapy, followed by oral isotretinoin. PBSC collection was done after two induction cycles. For purging, PBSC were mixed with carbonyl iron and phagocytic cells removed with samarium cobalt magnets. Remaining cells were mixed with immunomagnetic beads prepared with five monoclonal antibodies targeting neuroblastoma cell surface antigens and attached cells were removed using samarium cobalt magnets. Patients underwent autologous stem-cell transplantation with PBSC as randomly assigned after six cycles of induction therapy. The primary endpoint was event-free survival and was analysed by intention-to-treat. The trial is registered with ClinicalTrials.gov, number NCT00004188.
Findings: 495 patients were enrolled, of whom 486 were randomly assigned to treatment: 243 patients to receive non-purged PBSC and 243 to received purged PBSC. PBSC were collected from 229 patients from the purged group and 236 patients from the non-purged group, and 180 patients from the purged group and 192 from the non-purged group received transplant. 5-year event-free survival was 40% (95% CI 33-46) in the purged group versus 36% (30-42) in the non-purged group (p=0·77); 5-year overall survival was 50% (95% CI 43-56) in the purged group compared with 51% (44-57) in the non-purged group (p=0·81). Toxic deaths occurred in 15 patients during induction (eight in the purged group and seven in the non-purged group) and 12 during consolidation (eight in the purged group and four in the non-purged group). The most common adverse event reported was grade 3 or worse stomatitis during both induction (87 of 242 patients in the purged group and 93 of 243 patients in the non-purged group) and consolidation (131 of 177 in the purged group vs 145 of 191 in the non-purged group). Serious adverse events during induction were grade 3 or higher decreased cardiac function (four of 242 in the purged group and five of 243 in the non-purged group) and elevated creatinine (five of 242 in the purged group and six of 243 non-purged group) and during consolidation were sinusoidal obstructive syndrome (12 of 177 in the purged group and 17 of 191 in the non-purged group), acute vascular leak (11 of 177 in the purged group and nine of 191 in the non-purged group), and decreased cardiac function (one of 177 in the purged group and four of 191 in the non-purged group).
Interpretation: Immunomagnetic purging of PBSC for autologous stem-cell transplantation did not improve outcome, perhaps because of incomplete purging or residual tumour in patients. Non-purged PBSC are acceptable for support of myeloablative therapy of high-risk neuroblastoma.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
We declare that we have no conflicts of interest.
Figures
Figure 1. Treatment schema
PBSC=peripheral blood stem cells. AHSCT=autologous haemopoietic stem-cell transplantation. CEM=carboplatin, etoposide, and melphalan. GFR=glomerular filtration rate. AUC=area under the curve. G-CSF=granulocyte colony-stimulating factor. ANC=absolute neutrophil count. MIBG=123I or 131I- meta-iodobenzylguanidine.
Figure 2. Trial profile
*372 patients (192 non-purged, 180 purged) received a transplant, including those who received the PBSC product to which they were allocated (randomised) and those who were crossovers. †One additional patient who received a transplant was retrospectively (post-transplant) determined by the treating institution to have had progressive disease at the end of induction; thus table 1 presents a total of 38 patients in the purged group with end of induction progressive disease. ‡Patients alive at last contact were censored in survival analysis.
Figure 3. Event-free survival and overall survival
(A) Event-free survival for intention-to-treat population from time of enrolment or randomisation. (B) Overall survival for intention-to-treat population from time of enrolment or randomisation. (C) Event-free survival for comparison of patients randomly assigned to purged treatment group versus patients randomly assigned to non-purged treatment group, from time of transplantation. (D) Overall survival for comparison of patients randomly assigned to purged treatment group versus patients randomly assigned to non-purged treatment group, from time of transplantation.
Figure 4. Event-free survival and overall survival by TLDA test results
(A) Event-free survival. (B) Overall survival. TLDA=TaqMan low density array. PBSC=peripheral blood stem cells.
Comment in
- Role of purging in PBSC transplantation for neuroblastoma.
Klingebiel T. Klingebiel T. Lancet Oncol. 2013 Sep;14(10):919. doi: 10.1016/S1470-2045(13)70353-X. Epub 2013 Jul 25. Lancet Oncol. 2013. PMID: 23890778 No abstract available.
Similar articles
- Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): a multicentre, randomised, phase 3 trial.
Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Yaniv I, Laureys G, Brock P, Michon JM, Owens C, Trahair T, Chan GCF, Ruud E, Schroeder H, Beck Popovic M, Schreier G, Loibner H, Ambros P, Holmes K, Castellani MR, Gaze MN, Garaventa A, Pearson ADJ, Lode HN. Ladenstein R, et al. Lancet Oncol. 2018 Dec;19(12):1617-1629. doi: 10.1016/S1470-2045(18)30578-3. Epub 2018 Nov 12. Lancet Oncol. 2018. PMID: 30442501 Clinical Trial. - Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial.
Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, Simon T, Hero B. Berthold F, et al. Lancet Oncol. 2005 Sep;6(9):649-58. doi: 10.1016/S1470-2045(05)70291-6. Lancet Oncol. 2005. PMID: 16129365 Clinical Trial. - Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): an open-label, randomised, phase 2 trial.
Mody R, Naranjo A, Van Ryn C, Yu AL, London WB, Shulkin BL, Parisi MT, Servaes SE, Diccianni MB, Sondel PM, Bender JG, Maris JM, Park JR, Bagatell R. Mody R, et al. Lancet Oncol. 2017 Jul;18(7):946-957. doi: 10.1016/S1470-2045(17)30355-8. Epub 2017 May 23. Lancet Oncol. 2017. PMID: 28549783 Free PMC article. Clinical Trial. - High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.
Yalçin B, Kremer LC, Caron HN, van Dalen EC. Yalçin B, et al. Cochrane Database Syst Rev. 2013 Aug 22;(8):CD006301. doi: 10.1002/14651858.CD006301.pub3. Cochrane Database Syst Rev. 2013. PMID: 23970444 Updated. Review. - Retinoic acid post consolidation therapy for high-risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation.
Peinemann F, van Dalen EC, Tushabe DA, Berthold F. Peinemann F, et al. Cochrane Database Syst Rev. 2015 Jan 29;1:CD010685. doi: 10.1002/14651858.CD010685.pub2. Cochrane Database Syst Rev. 2015. PMID: 25634649 Updated. Review.
Cited by
- Mechanism of Drug Resistance to First-Line Chemotherapeutics Mediated by TXNDC17 in Neuroblastomas.
Zeng C, Li Z, Wei Z, Chen T, Wang J, Huang J, Sun F, Zhu J, Lu S, Zhen Z. Zeng C, et al. Cancer Rep (Hoboken). 2024 Oct;7(10):e70033. doi: 10.1002/cnr2.70033. Cancer Rep (Hoboken). 2024. PMID: 39411839 Free PMC article. - Epigenetic regulation of cell state by H2AFY governs immunogenicity in high-risk neuroblastoma.
Nagarajan D, Parracho RT, Corujo D, Xie M, Kutkaite G, Olsen TK, Rubies Bedos M, Salehi M, Baryawno N, Menden MP, Chen X, Buschbeck M, Mao Y. Nagarajan D, et al. J Clin Invest. 2024 Sep 10;134(21):e175310. doi: 10.1172/JCI175310. J Clin Invest. 2024. PMID: 39255035 Free PMC article. - Systemic inflammation enhances metastatic growth in a syngeneic neuroblastoma mouse model.
Mimura K, Fumino S, Yamashi K, Iguchi M, Inoue M, Takayama S, Kim K, Aoi S, Tajiri T, Ono S. Mimura K, et al. Pediatr Surg Int. 2024 Jul 17;40(1):195. doi: 10.1007/s00383-024-05788-9. Pediatr Surg Int. 2024. PMID: 39017743 - Extracting Electronic Health Record Neuroblastoma Treatment Data With High Fidelity Using the REDCap Clinical Data Interoperability Services Module.
Furner B, Cheng A, Desai AV, Benedetti DJ, Friedman DL, Wyatt KD, Watkins M, Volchenboum SL, Cohn SL. Furner B, et al. JCO Clin Cancer Inform. 2024 May;8:e2400009. doi: 10.1200/CCI.24.00009. JCO Clin Cancer Inform. 2024. PMID: 38815188 Free PMC article. - Attempts to Improve Lipophilic Drugs' Solubility and Bioavailability: A Focus on Fenretinide.
Alfei S, Zuccari G. Alfei S, et al. Pharmaceutics. 2024 Apr 24;16(5):579. doi: 10.3390/pharmaceutics16050579. Pharmaceutics. 2024. PMID: 38794242 Free PMC article. Review.
References
- Seeger RC, Reynolds CP, Gallego R, et al. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children’s Cancer Group Study. J Clin Oncol. 2000;18:4067–4076. - PubMed
- Rill DR, Santana VM, Roberts WM, et al. Direct demonstration that autologous bone marrow transplantation for solid tumors can return a multiplicity of tumorigenic cells. Blood. 1994;84:380–383. - PubMed
- Bensimhon P, Villablanca JG, Sender LS, et al. Peripheral blood stem cell support for multiple cycles of dose intensive induction therapy is feasible with little risk of tumor contamination in advanced stage neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;54:596–602. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- U10-CA98543/CA/NCI NIH HHS/United States
- U10 CA098413/CA/NCI NIH HHS/United States
- U10 CA029139/CA/NCI NIH HHS/United States
- R33-CA152809-01/CA/NCI NIH HHS/United States
- U10 CA030969/CA/NCI NIH HHS/United States
- U10-CA98413/CA/NCI NIH HHS/United States
- U10-CA29139/CA/NCI NIH HHS/United States
- U10 CA037379/CA/NCI NIH HHS/United States
- U10- CA30969/CA/NCI NIH HHS/United States
- R33 CA152809/CA/NCI NIH HHS/United States
- U10 CA098543/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical