Regulated membrane protein entry into flagella is facilitated by cytoplasmic microtubules and does not require IFT - PubMed (original) (raw)
Regulated membrane protein entry into flagella is facilitated by cytoplasmic microtubules and does not require IFT
Olivier Belzile et al. Curr Biol. 2013.
Abstract
The membrane protein composition of the primary cilium, a key sensory organelle, is dynamically regulated during cilium-generated signaling [1, 2]. During ciliogenesis, ciliary membrane proteins, along with structural and signaling proteins, are carried through the multicomponent, intensely studied ciliary diffusion barrier at the base of the organelle [3-8] by intraflagellar transport (IFT) [9-18]. A favored model is that signaling-triggered accumulation of previously excluded membrane proteins in fully formed cilia [19-21] also requires IFT, but direct evidence is lacking. Here, in studies of regulated entry of a membrane protein into the flagellum of Chlamydomonas, we show that cells use an IFT-independent mechanism to breach the diffusion barrier at the flagellar base. In resting cells, a flagellar signaling component [22], the integral membrane polypeptide SAG1-C65, is uniformly distributed over the plasma membrane and excluded from the flagellar membrane. Flagellar adhesion-induced signaling triggers rapid, striking redistribution of the protein to the apical ends of the cells concomitantly with entry into the flagella. Protein polarization and flagellar enrichment are facilitated by cytoplasmic microtubules. Using a conditional anterograde IFT mutant, we demonstrate that the IFT machinery is not required for regulated SAG1-C65 entry into flagella. Thus, integral membrane proteins can negotiate passage through the ciliary diffusion barrier without the need for a motor.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
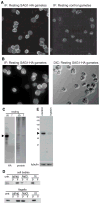
Figure 1. SAG1-HA is evenly distributed in the cell body plasma membrane and is expressed in gametes primarily as a 65 kDa C-terminal integral membrane polypeptide
(A and B) SAG1-C65-HA localizes to the cell body plasma membrane, with little on flagella and was not detectable in control hap2 gametes. (C) SAG1-C65-HA is expressed primarily as a 65 kDa C-terminal fragment and present in cell bodies with little in flagella. (D) Both flagellar and cell body SAG1-C65-HA are insoluble after freeze-thaw or incubation in 0.5 M NaCl, but are soluble in the non-ionic detergent, NP-40 (1%). (E) SAG1-C65-HA is degraded upon treatment of live gametes with 0.01% trypsin for 5 min (upper panel). The lower panel shows anti-tubulin staining.
Figure 2. During gamete activation, pre-existing SAG1-C65-HA rapidly moves from the cell body to the flagella
(A) SAG1-C65-HA becomes enriched in flagella during flagellar adhesion. The upper panel is an HA immunoblot and the lower panel shows the blot after staining for protein (9 μg protein/lane). (B) The SAG1-C65-HA that moves to flagella is derived from pre-existing SAG1-C65-HA. Immunoblots of cell bodies (7 × 105 cell equivalents [~7 μg protein] in each lane) and flagella (2 × 107 cell equivalents, [~3 μg protein] in each lane) isolated from SAG1-HA gametes mixed with hap2 minus gametes in the presence and absence of 10 μg/mL cycloheximide for the indicated times. Cells were pretreated for 30 min with CH before mixing. (C) Signaling is necessary and sufficient for rapid enrichment of SAG1-C65-HA in flagella. SAG1-C65-HA becomes enriched in flagella upon incubation of SAG1-HA gametes with db-cAMP (left panel; flagella from equal numbers of cells loaded in each lane). SAG1-HA gametes mixed with minus gametes in the presence of 1 μM staurosporine fail to enrich SAG1-C65-HA in their flagella (right panel; flagella from equal numbers of cells loaded in each lane). (D) Immunolocalization experiments show that SAG1-C65-HA becomes enriched in flagella of SAG1-HA gametes activated by incubation in db-cAMP (30 min).
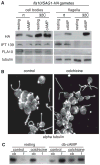
Figure 3. Signaling-induced flagellar enrichment of SAG1-C65-HA is independent of IFT and facilitated by cytoplasmic microtubules
(A) Immunoblots with the indicated antibodies of cell bodies and flagella (5 μg protein/lane) of SAG1-HA/ fla10 resting and activated gametes that had been incubated at the permissive and non-permissive temperatures for 45 minutes and then activated with db-cAMP for 5 min. (B) Incubation of cells for 100 min in 2 mg/ml colchicine disrupts the extensive array of cytoplasmic microtubules (right panel, control cells; left panel, colchicine treated cells; images are 2D projections of Z-stacks of confocal images of samples stained with anti-tubulin antibody). (C). Depletion of cytoplasmic microtubules impairs db-cAMP-induced flagellar enrichment of SAG1-C65-HA (10 min treatment with db-cAMP; 2 μg protein/lane).
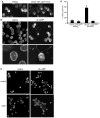
Figure 4. Gamete activation triggers SAG1-C65-HA redistribution to the apical ends of the cells near the site of entry into flagella and polarization depends on cytoplasmic microtubules
(A and B) Signaling induced by flagellar adhesion (A) (20 min mixing with hap2 minus gametes) or by 10 min incubation with db-cAMP (B) is accompanied by enrichment of SAG1-C65-HA at the apical plasma membrane. The lower panels in B are higher magnification views. (C and D) Apical enrichment is inhibited when cytoplasmic microtubules are disrupted by pre-incubation in colchicine. Upper left and right panels in (C) are control resting and db-cAMP-treated cells (respectively). Lower left and right panels in (C) are colchicine-treated resting and db-cAMP-treated cells (respectively). For the quantification shown in (D), a cell was scored as having apical accumulation of SAG1-C65-HA if the protein was depleted from the ~ lower 2/3 of the cell body plasma membrane and was concentrated at the apical end of the cell. Data are from 3 experiments; 100 cells were scored for each condition. Error bars indicate +/− SEM.
Similar articles
- Transient Internalization and Microtubule-Dependent Trafficking of a Ciliary Signaling Receptor from the Plasma Membrane to the Cilium.
Ranjan P, Awasthi M, Snell WJ. Ranjan P, et al. Curr Biol. 2019 Sep 9;29(17):2942-2947.e2. doi: 10.1016/j.cub.2019.07.022. Epub 2019 Aug 15. Curr Biol. 2019. PMID: 31422889 Free PMC article. - Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding.
Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ. Cao M, et al. Elife. 2015 Feb 17;4:e05242. doi: 10.7554/eLife.05242. Elife. 2015. PMID: 25688564 Free PMC article. - Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas.
Wang Q, Pan J, Snell WJ. Wang Q, et al. Cell. 2006 May 5;125(3):549-62. doi: 10.1016/j.cell.2006.02.044. Cell. 2006. PMID: 16678098 - The intraflagellar transport machinery of Chlamydomonas reinhardtii.
Cole DG. Cole DG. Traffic. 2003 Jul;4(7):435-42. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. Traffic. 2003. PMID: 12795688 Review. - Architecture of the IFT ciliary trafficking machinery and interplay between its components.
Nakayama K, Katoh Y. Nakayama K, et al. Crit Rev Biochem Mol Biol. 2020 Apr;55(2):179-196. doi: 10.1080/10409238.2020.1768206. Epub 2020 May 26. Crit Rev Biochem Mol Biol. 2020. PMID: 32456460 Review.
Cited by
- A cytoplasmic protein kinase couples engagement of Chlamydomonas ciliary receptors to cAMP-dependent cellular responses.
Awasthi M, Ranjan P, Kelterborn S, Hegemann P, Snell WJ. Awasthi M, et al. J Cell Sci. 2022 May 15;135(10):jcs259814. doi: 10.1242/jcs.259814. Epub 2022 May 23. J Cell Sci. 2022. PMID: 35502650 Free PMC article. - Cargo adapters expand the transport range of intraflagellar transport.
Lechtreck K. Lechtreck K. J Cell Sci. 2022 Dec 15;135(24):jcs260408. doi: 10.1242/jcs.260408. Epub 2022 Dec 19. J Cell Sci. 2022. PMID: 36533425 Free PMC article. Review. - Protein transport in growing and steady-state cilia.
Lechtreck KF, Van De Weghe JC, Harris JA, Liu P. Lechtreck KF, et al. Traffic. 2017 May;18(5):277-286. doi: 10.1111/tra.12474. Epub 2017 Mar 29. Traffic. 2017. PMID: 28248449 Free PMC article. Review. - KIF13B establishes a CAV1-enriched microdomain at the ciliary transition zone to promote Sonic hedgehog signalling.
Schou KB, Mogensen JB, Morthorst SK, Nielsen BS, Aleliunaite A, Serra-Marques A, Fürstenberg N, Saunier S, Bizet AA, Veland IR, Akhmanova A, Christensen ST, Pedersen LB. Schou KB, et al. Nat Commun. 2017 Jan 30;8:14177. doi: 10.1038/ncomms14177. Nat Commun. 2017. PMID: 28134340 Free PMC article. - Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism.
Craft JM, Harris JA, Hyman S, Kner P, Lechtreck KF. Craft JM, et al. J Cell Biol. 2015 Jan 19;208(2):223-37. doi: 10.1083/jcb.201409036. Epub 2015 Jan 12. J Cell Biol. 2015. PMID: 25583998 Free PMC article.
References
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2012;14:61–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources