Experimental cross-species infection of common marmosets by titi monkey adenovirus - PubMed (original) (raw)
. 2013 Jul 24;8(7):e68558.
doi: 10.1371/journal.pone.0068558. Print 2013.
Shigeo Yagi, Ricardo Carrion Jr, Eunice C Chen, Maria Liu, Kathleen M Brasky, Robert E Lanford, Kristi R Kelly, Karen L Bales, David P Schnurr, Don R Canfield, Jean L Patterson, Charles Y Chiu
Affiliations
- PMID: 23894316
- PMCID: PMC3722195
- DOI: 10.1371/journal.pone.0068558
Experimental cross-species infection of common marmosets by titi monkey adenovirus
Guixia Yu et al. PLoS One. 2013.
Abstract
Adenoviruses are DNA viruses that infect a number of vertebrate hosts and are associated with both sporadic and epidemic disease in humans. We previously identified a novel adenovirus, titi monkey adenovirus (TMAdV), as the cause of a fulminant pneumonia outbreak in a colony of titi monkeys (Callicebus cupreus) at a national primate center in 2009. Serological evidence of infection by TMAdV was also found in a human researcher at the facility and household family member, raising concerns for potential cross-species transmission of the virus. Here we present experimental evidence of cross-species TMAdV infection in common marmosets (Callithrix jacchus). Nasal inoculation of a cell cultured-adapted TMAdV strain into three marmosets produced an acute, mild respiratory illness characterized by low-grade fever, reduced activity, anorexia, and sneezing. An increase in virus-specific neutralization antibody titers accompanied the development of clinical signs. Although serially collected nasal swabs were positive for TMAdV for at least 8 days, all 3 infected marmosets spontaneously recovered by day 12 post-inoculation, and persistence of the virus in tissues could not be established. Thus, the pathogenesis of experimental inoculation of TMAdV in common marmosets resembled the mild, self-limiting respiratory infection typically seen in immunocompetent human hosts rather than the rapidly progressive, fatal pneumonia observed in 19 of 23 titi monkeys during the prior 2009 outbreak. These findings further establish the potential for adenovirus cross-species transmission and provide the basis for development of a monkey model useful for assessing the zoonotic potential of adenoviruses.
Conflict of interest statement
Competing Interests: This study was partly funded by an Abbott Viral Discovery Award. Charles Y. Chiu is the director of the UCSF-Abbott Viral Diagnostics and Discovery Center (VDDC) and Guixia Yu is employed by the VDDC. The University of California has also filed a patent application related to TMAdV (US Patent Application 20130034576A1). This does not alter the authors’ adherence to all PLOS ONE policies on sharing data and materials.
Figures
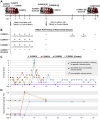
Figure 1. Whole-Genome Sequencing of Serially Passaged TMAdV.
(A) Genomic coverage of TMAdV by mapping of deep sequencing reads from a lung swab from a titi monkey with TMAdV pneumonia, passage 4 in A549 cells, and passage 10 in A549 cells. The coverage (y-axis) achieved at each position along the ∼37 kB TMAdV genome (x-axis) is plotted on a logarithmic scale. (B) Identification of single nucleotide polymorphisms (SNPs) in assembled TMAdV genomes. The coverage at each SNP position is shown in parentheses. SNP positions 14776A and 36545A (cyan lollipops) correspond to probable sequencing errors in the Sanger sequenced TMAdV genome (Genbank HQ913600). A 3202CG mutation is observed to occur between passage 4 (black lollipop) and passage 10 (red lollipop), which results in a 388PR amino acid coding change in the TMAdV E1B-55K protein. (C) A multiple sequence alignment of the E1B-55K protein of TMAdV and representative human and simian adenoviruses reveals that position 388 resides within a putative binding region for p53 and/or the MRE11-RAD50-NBS1 (MRN) complex. Human adenovirus type 5 (HAdV-5) mutants containing a nearby H534 insertion or mutations in the downstream C2H2 zinc finger inhibit binding to p53 and/or MRN .
Figure 2. Experimental TMAdV Infection in the Common Marmoset.
(A) Outline of TMAdV infection protocol and sample collections. The “[X]” refers to a marmoset that is sacrificed at the designated timepoint. (B) Results from PCR analysis of nasal swab samples collected at serial timepoints. Shown in parentheses are the calculated viral titers in genome copy numbers per mL associated with each timepoint. (C) Clinical scores in TMAdV-infected and control marmosets. The asterisks (*) refer to timepoints during which each infected marmoset exhibited the most pronounced clinical signs of illness (inset box). For a definition of the clinical scoring system, see Table S2. (D) Antibody titers in TMAdV-infected and control marmosets as measured using a TMAdV neutralization assay.
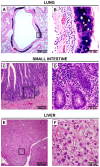
Figure 3. TMAdV-Associated Pathology in Experimentally Infected Marmoset CJ29019.
Histologic findings on necropsy tissue from day 15 post-inoculation include a mild bronchitis (A and B), enteritis (C and D), and an atypical nodular hyperplasia in the liver (E and F). The black rectangle in panels A, C, or E outlines the region magnified in panels B, D, or F, respectively.
Figure 4. TMAdV Antibody Staining of Titi and Marmoset Monkey Tissues.
Direct immunofluorescence staining of lung from two TMAdV-infected titi monkeys with fatal pneumonia (A-B, with corresponding magnified views in C-D) , and lung and liver from two TMAdV-inoculated marmosets (E-H) are shown. TMAdV staining is evident in lung from naturally infected titi monkeys who died from pneumonia, but absent in lung and liver from experimentally infected marmosets.
Similar articles
- Epidemiological and molecular characterization of a novel adenovirus of squirrel monkeys after fatal infection during immunosuppression.
Rogers DL, Ruiz JC, Baze WB, McClure GB, Smith C, Urbanowski R, Boston T, Simmons JH, Williams L, Abee CR, Vanchiere JA. Rogers DL, et al. Microb Genom. 2020 Sep;6(9):mgen000395. doi: 10.1099/mgen.0.000395. Epub 2020 Jul 2. Microb Genom. 2020. PMID: 32614763 Free PMC article. - Cross-species transmission of a novel adenovirus associated with a fulminant pneumonia outbreak in a new world monkey colony.
Chen EC, Yagi S, Kelly KR, Mendoza SP, Tarara RP, Canfield DR, Maninger N, Rosenthal A, Spinner A, Bales KL, Schnurr DP, Lerche NW, Chiu CY. Chen EC, et al. PLoS Pathog. 2011 Jul;7(7):e1002155. doi: 10.1371/journal.ppat.1002155. Epub 2011 Jul 14. PLoS Pathog. 2011. PMID: 21779173 Free PMC article. - A novel adenovirus species associated with an acute respiratory outbreak in a baboon colony and evidence of coincident human infection.
Chiu CY, Yagi S, Lu X, Yu G, Chen EC, Liu M, Dick EJ Jr, Carey KD, Erdman DD, Leland MM, Patterson JL. Chiu CY, et al. mBio. 2013 Apr 16;4(2):e00084. doi: 10.1128/mBio.00084-13. mBio. 2013. PMID: 23592261 Free PMC article. - Waterborne adenovirus.
Mena KD, Gerba CP. Mena KD, et al. Rev Environ Contam Toxicol. 2009;198:133-67. doi: 10.1007/978-0-387-09647-6_4. Rev Environ Contam Toxicol. 2009. PMID: 19253037 Review. - [Adenovirus infection in immunocompromised patients].
Rynans S, Dzieciątkowski T, Młynarczyk G. Rynans S, et al. Postepy Hig Med Dosw (Online). 2013 Sep 11;67:964-72. doi: 10.5604/17322693.1066199. Postepy Hig Med Dosw (Online). 2013. PMID: 24088540 Review. Polish.
Cited by
- What is the risk of a deadly adenovirus pandemic?
Kremer EJ. Kremer EJ. PLoS Pathog. 2021 Sep 2;17(9):e1009814. doi: 10.1371/journal.ppat.1009814. eCollection 2021 Sep. PLoS Pathog. 2021. PMID: 34473804 Free PMC article. - Marmosets as a preclinical model for testing "off-label" use of doxycycline to turn on Flt3L expression from high-capacity adenovirus vectors.
VanderVeen N, Paran C, Appelhans A, Krasinkiewicz J, Lemons R, Appelman H, Doherty R, Palmer D, Ng P, Lowenstein PR, Castro MG. VanderVeen N, et al. Mol Ther Methods Clin Dev. 2014 Feb 5;1:10-. doi: 10.1038/mtm.2013.10. Mol Ther Methods Clin Dev. 2014. PMID: 25068145 Free PMC article. - Adenovirus in Rural Côte D'Ivoire: High Diversity and Cross-Species Detection.
Pauly M, Akoua-Koffi C, Buchwald N, Schubert G, Weiss S, Couacy-Hymann E, Anoh AE, Mossoun A, Calvignac-Spencer S, Leendertz SA, Leendertz FH, Ehlers B. Pauly M, et al. Ecohealth. 2015 Sep;12(3):441-52. doi: 10.1007/s10393-015-1032-5. Epub 2015 May 20. Ecohealth. 2015. PMID: 25990885 - High Diversity and Novel Enteric Viruses in Fecal Viromes of Healthy Wild and Captive Thai Cynomolgus Macaques (Macaca fascicularis).
Sawaswong V, Fahsbender E, Altan E, Kemthong T, Deng X, Malaivijitnond S, Payungporn S, Delwart E. Sawaswong V, et al. Viruses. 2019 Oct 22;11(10):971. doi: 10.3390/v11100971. Viruses. 2019. PMID: 31652508 Free PMC article. - Epidemiological and molecular characterization of a novel adenovirus of squirrel monkeys after fatal infection during immunosuppression.
Rogers DL, Ruiz JC, Baze WB, McClure GB, Smith C, Urbanowski R, Boston T, Simmons JH, Williams L, Abee CR, Vanchiere JA. Rogers DL, et al. Microb Genom. 2020 Sep;6(9):mgen000395. doi: 10.1099/mgen.0.000395. Epub 2020 Jul 2. Microb Genom. 2020. PMID: 32614763 Free PMC article.
References
- Harrach B, Benkö M, Both G, Brown M, Davis A, et al.. (2011) Family Adenoviridae. In: King A, Adams M, Carstens E, Lefkowitz E, editors. Virus Taxonomy: Classification and Nomenclature of Viruses Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier. 95–111.
- Lewis PF, Schmidt MA, Lu X, Erdman DD, Campbell M, et al. (2009) A community-based outbreak of severe respiratory illness caused by human adenovirus serotype 14. J Infect Dis 199: 1427–1434. - PubMed
- Wold W, Horwitz M (2007) Adenoviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2395–2436.
- Tsai JC, Garlinghouse G, McDonnell PJ, Trousdale MD (1992) An experimental animal model of adenovirus-induced ocular disease. The cotton rat. Arch Ophthalmol 110: 1167–1170. - PubMed
Publication types
MeSH terms
Grants and funding
- U54 AI057156/AI/NIAID NIH HHS/United States
- P51 OD011107/OD/NIH HHS/United States
- R56-AI089532/AI/NIAID NIH HHS/United States
- R01-HL105704/HL/NHLBI NIH HHS/United States
- U54-AI57168/AI/NIAID NIH HHS/United States
- P51-0D011107/PHS HHS/United States
- R56 AI089532/AI/NIAID NIH HHS/United States
- U54-AI057156/AI/NIAID NIH HHS/United States
- P51 OD011133/OD/NIH HHS/United States
- U54 AI057168/AI/NIAID NIH HHS/United States
- R01 HL105704/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources