Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies - PubMed (original) (raw)
Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies
Yannick Bulliard et al. J Exp Med. 2013.
Abstract
Fc γ receptor (FcγR) coengagement can facilitate antibody-mediated receptor activation in target cells. In particular, agonistic antibodies that target tumor necrosis factor receptor (TNFR) family members have shown dependence on expression of the inhibitory FcγR, FcγRIIB. It remains unclear if engagement of FcγRIIB also extends to the activities of antibodies targeting immunoregulatory TNFRs expressed by T cells. We have explored the requirement for activating and inhibitory FcγRs for the antitumor effects of antibodies targeting the TNFR glucocorticoid-induced TNFR-related protein (GITR; TNFRSF18; CD357) expressed on activated and regulatory T cells (T reg cells). We found that although FcγRIIB was dispensable for the in vivo efficacy of anti-GITR antibodies, in contrast, activating FcγRs were essential. Surprisingly, the dependence on activating FcγRs extended to an antibody targeting the non-TNFR receptor CTLA-4 (CD152) that acts as a negative regulator of T cell immunity. We define a common mechanism that correlated with tumor efficacy, whereby antibodies that coengaged activating FcγRs expressed by tumor-associated leukocytes facilitated the selective elimination of intratumoral T cell populations, particularly T reg cells. These findings may have broad implications for antibody engineering efforts aimed at enhancing the therapeutic activity of immunomodulatory antibodies.
Figures
Figure 1.
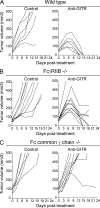
Activating, rather than inhibitory, FcγRs are necessary for the antitumor activity of an agonistic antibody to GITR. Efficacy study of anti-GITR antibody (DTA-1 rIgG2b; 5 mg/kg i.p.) in wild type (A), FcγRIIB−/− (B), and Fc common γ chain−/− (C) BALB/c mice bearing Colon26 tumors (n = 6–10 mice per treatment group). Day 0 refers to treatment day, 6–8 d after tumor inoculation. Data is a representative of two or more independent experiments.
Figure 2.
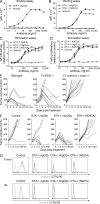
Engagement of FcγRs by DTA-1 is required for antitumor activity. (A and B) GITR-binding assay. Primary splenocytes stimulated with CD3- and CD28-specific antibodies served as targets. DTA-1 variants were detected using rat IgG2b-specific (A) or murine IgG2a-specific (B) PE-conjugated antibodies. (C and D) In vitro activity of GITR-specific antibodies at various concentrations tested on GITR-expressing NF-kB 293 reporter cell line (C) and splenocytes incubated with suboptimal doses of anti-CD3 and anti-CD28 antibodies (D). The in vitro data are derived from triplicates and are a representative of two or more independent experiments. (E) Efficacy study of 5 mg/kg DTA-1 mIgG2a in wild-type, FcγRIIB−/−, and Fc common γ chain−/− mice bearing Colon26 tumors (n = 7–10 mice per treatment group). Mean and standard errors are based on triplicates, and the data is a representative of two or more independent experiments. (F) In vivo efficacy study after treatment with the DTA-1 variant antibodies (n = 7). The efficacy data are a representative of two or more independent experiments. (G) Saturation of GITR on T reg cells in the tumor and draining lymph node by the three versions of DTA-1.
Figure 3.
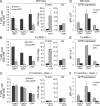
Engagement of FcγRs by DTA-1 induces loss of intratumoral T reg cells early after treatment. (A) Cell surface expression of GITR on day 0 (n = 3). (B and C) Dot plots of CD11b+ myeloid and DX5+ NK cells, gated on live CD45+ leukocytes (B) and expression of FcγRII/III and IV by the two cell populations in the tumor (C). (D) Dot plots of T cells 3 d after treatment with the DTA-1 variant antibodies. Percentages of live CD45+ CD3+ T cells are indicated. The data are a representative of two or more independent experiments. (E and F) Density of T reg cells (E) and FoxP3-CD4+ T cells and CD8+ T cells (F) in the tumor after treatment with the 5 mg/kg DTA-1 variants. (G) Ratio between CD8+ T cells and T reg cells in the tumor. (H) Percentage of intratumoral CD45+ leukocytes. (I) T cell number in the draining lymph nodes after treatment with 5 mg/kg of the DTA-1 variants. Mean and standard errors are based on triplicates from two independent experiments. P-values were calculated using an unpaired Student’s t test (**, P < 0.001).
Figure 4.
Activating, but not inhibitory, FcγRs are required for intratumoral T reg cell depletion by antibodies targeting GITR. Intratumoral T cell density and CD8+ T cells to T reg cells ratios 5 d after treatment with 5 mg/kg DTA-1-mIgG2a using wild-type (A), FcγRIIB−/− (B), or Fc common γ chain−/− (C) mice bearing Colon26 tumors. (D–F) Cell surface expression of GITR on T cells. Mean and standard errors are based on triplicates from two independent experiments. P-values were calculated using an unpaired Student’s t test (**, P < 0.001).
Figure 5.
Engagement of activating FcγRs is also required for the antitumor activity of an antibody targeting the non-TNFR CTLA-4. (A) Efficacy study using 15 mg/kg CTLA-4–specific antibody (9D9) in wild-type (top) or Fc common γ chain−/− (middle) mice bearing Colon26 tumors (n = 8–10 mice per treatment group). (B) Intratumoral T cell density and CD8+ to T reg cells ratios 5 d after treatment. (C) Cell surface expression of CTLA-4 on T cells. The efficacy data are a representative of two or more independent experiments. Mean and standard errors are based on triplicates from two independent experiments. P-values were calculated using an unpaired Student’s t test (*, P < 0.01).
Similar articles
- OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy.
Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. Bulliard Y, et al. Immunol Cell Biol. 2014 Jul;92(6):475-80. doi: 10.1038/icb.2014.26. Epub 2014 Apr 15. Immunol Cell Biol. 2014. PMID: 24732076 - Fcγ receptors enable anticancer action of proapoptotic and immune-modulatory antibodies.
Kim JM, Ashkenazi A. Kim JM, et al. J Exp Med. 2013 Aug 26;210(9):1647-51. doi: 10.1084/jem.20131625. J Exp Med. 2013. PMID: 23980122 Free PMC article. Review. - Authentic GITR Signaling Fails To Induce Tumor Regression unless Foxp3+ Regulatory T Cells Are Depleted.
Kim YH, Shin SM, Choi BK, Oh HS, Kim CH, Lee SJ, Kim KH, Lee DG, Park SH, Kwon BS. Kim YH, et al. J Immunol. 2015 Nov 15;195(10):4721-9. doi: 10.4049/jimmunol.1403076. Epub 2015 Sep 30. J Immunol. 2015. PMID: 26423152 - CD27-Mediated Regulatory T Cell Depletion and Effector T Cell Costimulation Both Contribute to Antitumor Efficacy.
Wasiuk A, Testa J, Weidlick J, Sisson C, Vitale L, Widger J, Crocker A, Thomas LJ, Goldstein J, Marsh HC, Keler T, He LZ. Wasiuk A, et al. J Immunol. 2017 Dec 15;199(12):4110-4123. doi: 10.4049/jimmunol.1700606. Epub 2017 Nov 6. J Immunol. 2017. PMID: 29109120 Free PMC article. - Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies.
Furness AJ, Vargas FA, Peggs KS, Quezada SA. Furness AJ, et al. Trends Immunol. 2014 Jul;35(7):290-8. doi: 10.1016/j.it.2014.05.002. Epub 2014 Jun 18. Trends Immunol. 2014. PMID: 24953012 Review.
Cited by
- Bi-specific antibody engagers for cancer immunotherapy.
Ploegh H, Liu X, Le Gall C, Alexander R, Borgman E, Balligand T. Ploegh H, et al. Res Sq [Preprint]. 2024 Aug 1:rs.3.rs-4792057. doi: 10.21203/rs.3.rs-4792057/v1. Res Sq. 2024. PMID: 39149504 Free PMC article. Preprint. - Targeting cytotoxic lymphocyte antigen 4 (CTLA-4) in breast cancer.
Jama M, Tabana Y, Barakat KH. Jama M, et al. Eur J Med Res. 2024 Jul 2;29(1):353. doi: 10.1186/s40001-024-01901-9. Eur J Med Res. 2024. PMID: 38956700 Free PMC article. Review. - Janus kinase inhibitor overcomes resistance to immune checkpoint inhibitor treatment in peritoneal dissemination of gastric cancer in C57BL/6 J mice.
Du WY, Masuda H, Nagaoka K, Yasuda T, Kuge K, Seto Y, Kakimi K, Nomura S. Du WY, et al. Gastric Cancer. 2024 Sep;27(5):971-985. doi: 10.1007/s10120-024-01514-5. Epub 2024 May 28. Gastric Cancer. 2024. PMID: 38805119 Free PMC article. - In vivo Auto-tuning of Antibody-Drug Conjugate Delivery for Effective Immunotherapy using High-Avidity, Low-Affinity Antibodies.
Kopp A, Dong S, Kwon H, Wang T, Desai AA, Linderman JJ, Tessier P, Thurber GM. Kopp A, et al. bioRxiv [Preprint]. 2024 Apr 10:2024.04.06.588433. doi: 10.1101/2024.04.06.588433. bioRxiv. 2024. PMID: 38645231 Free PMC article. Preprint. - Perspective view of allogeneic IgG tumor immunotherapy.
Liu Y, Huang Y, Cui HW, Wang Y, Ma Z, Xiang Y, Xin HY, Liang JQ, Xin HW. Liu Y, et al. Cancer Cell Int. 2024 Mar 9;24(1):100. doi: 10.1186/s12935-024-03290-9. Cancer Cell Int. 2024. PMID: 38461238 Free PMC article. Review.
References
- Albanesi M., Mancardi D.A., Macdonald L.E., Iannascoli B., Zitvogel L., Murphy A.J., Daëron M., Leusen J.H., Bruhns P. 2012. Cutting edge: FcγRIII (CD16) and FcγRI (CD64) are responsible for anti-glycoprotein 75 monoclonal antibody TA99 therapy for experimental metastatic B16 melanoma. J. Immunol. 189:5513–5517 10.4049/jimmunol.1201511 - DOI - PubMed
- Chung K.Y., Gore I., Fong L., Venook A., Beck S.B., Dorazio P., Criscitiello P.J., Healey D.I., Huang B., Gomez-Navarro J., Saltz L.B. 2010. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J. Clin. Oncol. 28:3485–3490 10.1200/JCO.2010.28.3994 - DOI - PubMed
- Cohen A.D., Schaer D.A., Liu C., Li Y., Hirschhorn-Cymmerman D., Kim S.C., Diab A., Rizzuto G., Duan F., Perales M.A., et al. 2010. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE. 5:e10436 10.1371/journal.pone.0010436 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases