Intravenous magnetic nanoparticle cancer hyperthermia - PubMed (original) (raw)
Intravenous magnetic nanoparticle cancer hyperthermia
Hui S Huang et al. Int J Nanomedicine. 2013.
Abstract
Magnetic nanoparticles heated by an alternating magnetic field could be used to treat cancers, either alone or in combination with radiotherapy or chemotherapy. However, direct intratumoral injections suffer from tumor incongruence and invasiveness, typically leaving undertreated regions, which lead to cancer regrowth. Intravenous injection more faithfully loads tumors, but, so far, it has been difficult achieving the necessary concentration in tumors before systemic toxicity occurs. Here, we describe use of a magnetic nanoparticle that, with a well-tolerated intravenous dose, achieved a tumor concentration of 1.9 mg Fe/g tumor in a subcutaneous squamous cell carcinoma mouse model, with a tumor to non-tumor ratio > 16. With an applied field of 38 kA/m at 980 kHz, tumors could be heated to 60°C in 2 minutes, durably ablating them with millimeter (mm) precision, leaving surrounding tissue intact.
Keywords: alternating magnetic field; cancer; hyperthermia; intravenous delivery; magnetic nanoparticles.
Figures
Figure 1
Transmission electron microscopy images of the magnetic nanoparticles. (A) Particle cores measured to be 11.3 ± 2.3 nm (scale bar = 100 nm). (B) High-resolution lattice image of a 9.9 nm particle showing its crystalline core (scale bar = 5 nm). (C) Electron diffraction pattern, identifying cores as Fe3O4 (scale bar = 10 nm−1).
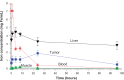
Figure 2
Biodistribution of iron (after subtraction of normal tissue iron) over time. Notes: The maximum iron concentration in the tumor from the points measured was at 24 hours post-injection, reaching 1.9 mg Fe/mL. Time points were: 5 minutes, 1 hour, and 4, 8, 24, and 96 hours.
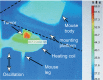
Figure 3
Thermal image of subcutaneous tumor being heated by an alternating magnetic field. Notes: The leg was scanned up and down vertically to make the field uniform over the leg. The tumor can be observed to have specifically heated (red region).
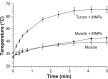
Figure 4
Heating of tissues in the magnetic field: 24 hours after an intravenous injection of magnetic nanoparticles (MNPs) (1.7 g Fe/kg) – tumor (filled circles) and leg muscle (no tumor, filled squares) tissues. Notes: Also shown is heating of leg muscle tissue with no injection of MNPs (open circles). The alternating magnetic field applied was 38 kA/m at 980 kHz. Tumors (average size of 206 mm3, three averaged) equilibrated at 66°C after 5 minutes, muscle with MNPs reached 42°C, and muscle without MNPs reached 39°C. Three mice were used per group.
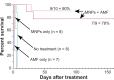
Figure 5
Magnetic nanoparticle (MNP) hyperthermia treatment. Notes: Intravenous injection of 1.7 g Fe/kg and followed 24 hours later by exposure to an alternating magnetic field (38 kA/m, 980 kHz, 2 minutes) resulted in durable ablation of tumors (7/9 = 78%, n = 9, absence of palpable tumor). A repeated experiment showed 90% (n = 10) thermoablation. Controls – no treatment, magnetic field only, and only magnetic nanoparticles – had no measurable effect on survival. Abbreviations: AMF, alternating magnetic field; n, number of animals per group.
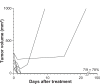
Figure 6
There was a rapid decrement in the volume of most tumors and debris was resorbed in 1–2 days, completely deflating fairly large tumors. Note: The average tumor size at time of treatment was 196 mm3.
Similar articles
- Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia.
Albarqi HA, Wong LH, Schumann C, Sabei FY, Korzun T, Li X, Hansen MN, Dhagat P, Moses AS, Taratula O, Taratula O. Albarqi HA, et al. ACS Nano. 2019 Jun 25;13(6):6383-6395. doi: 10.1021/acsnano.8b06542. Epub 2019 May 17. ACS Nano. 2019. PMID: 31082199 Free PMC article. - Real-time infrared thermography detection of magnetic nanoparticle hyperthermia in a murine model under a non-uniform field configuration.
Rodrigues HF, Mello FM, Branquinho LC, Zufelato N, Silveira-Lacerda EP, Bakuzis AF. Rodrigues HF, et al. Int J Hyperthermia. 2013 Dec;29(8):752-67. doi: 10.3109/02656736.2013.839056. Epub 2013 Oct 18. Int J Hyperthermia. 2013. PMID: 24138472 - Characterization of intratumor magnetic nanoparticle distribution and heating in a rat model of metastatic spine disease.
Zadnik PL, Molina CA, Sarabia-Estrada R, Groves ML, Wabler M, Mihalic J, McCarthy EF, Gokaslan ZL, Ivkov R, Sciubba D. Zadnik PL, et al. J Neurosurg Spine. 2014 Jun;20(6):740-50. doi: 10.3171/2014.2.SPINE13142. Epub 2014 Apr 4. J Neurosurg Spine. 2014. PMID: 24702509 - Cancer hyperthermia using magnetic nanoparticles.
Kobayashi T. Kobayashi T. Biotechnol J. 2011 Nov;6(11):1342-7. doi: 10.1002/biot.201100045. Epub 2011 Aug 26. Biotechnol J. 2011. PMID: 22069094 Review. - Thermal potentiation of chemotherapy by magnetic nanoparticles.
Torres-Lugo M, Rinaldi C. Torres-Lugo M, et al. Nanomedicine (Lond). 2013 Oct;8(10):1689-707. doi: 10.2217/nnm.13.146. Nanomedicine (Lond). 2013. PMID: 24074390 Free PMC article. Review.
Cited by
- Recent advances in nanotheranostics for triple negative breast cancer treatment.
Thakur V, Kutty RV. Thakur V, et al. J Exp Clin Cancer Res. 2019 Oct 28;38(1):430. doi: 10.1186/s13046-019-1443-1. J Exp Clin Cancer Res. 2019. PMID: 31661003 Free PMC article. Review. - Magnetic core-shell Fe3 O4 @TiO2 nanocomposites for broad spectrum antibacterial applications.
Rani N, Dehiya BS. Rani N, et al. IET Nanobiotechnol. 2021 May;15(3):301-308. doi: 10.1049/nbt2.12017. IET Nanobiotechnol. 2021. PMID: 34694669 Free PMC article. - Magnetic Hyperthermia for Cancer Treatment: Main Parameters Affecting the Outcome of In Vitro and In Vivo Studies.
Vilas-Boas V, Carvalho F, Espiña B. Vilas-Boas V, et al. Molecules. 2020 Jun 22;25(12):2874. doi: 10.3390/molecules25122874. Molecules. 2020. PMID: 32580417 Free PMC article. Review. - Real-time in vivo monitoring of magnetic nanoparticles in the bloodstream by AC biosusceptometry.
Próspero AG, Quini CC, Bakuzis AF, Fidelis-de-Oliveira P, Moretto GM, Mello FP, Calabresi MF, Matos RV, Zandoná EA, Zufelato N, Oliveira RB, Miranda JR. Próspero AG, et al. J Nanobiotechnology. 2017 Mar 21;15(1):22. doi: 10.1186/s12951-017-0257-6. J Nanobiotechnology. 2017. PMID: 28327191 Free PMC article. - Prostate-specific membrane antigen (PSMA)-targeted photodynamic therapy enhances the delivery of PSMA-targeted magnetic nanoparticles to PSMA-expressing prostate tumors.
Ngen EJ, Chen Y, Azad BB, Boinapally S, Jacob D, Lisok A, Shen C, Hossain MS, Jin J, Bhujwalla ZM, Pomper MG, Banerjee SR. Ngen EJ, et al. Nanotheranostics. 2021 Jan 19;5(2):182-196. doi: 10.7150/ntno.52361. eCollection 2021. Nanotheranostics. 2021. PMID: 33564617 Free PMC article.
References
- Hergt R, Dutz S, Röder M. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. J Phys Condens Matter. 2008;20(38):385214. - PubMed
- Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater. 2002;252(1–3):370–374.
- Chan DC, Kirpotin DB, Bunn PA., Jr Synthesis and evaluation of colloidal magnetic iron oxides for the site-specific radiofrequency-induced hyperthermia of cancer. J Magn Magn Mater. 1993;122(1–3):374–378.
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: physical evaluation of their potential for hyperthermia. Int J Hyperthermia. 1993;9(1):51–68. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources