Regulation of N-methyl-D-aspartate receptors by disrupted-in-schizophrenia-1 - PubMed (original) (raw)
Regulation of N-methyl-D-aspartate receptors by disrupted-in-schizophrenia-1
Jing Wei et al. Biol Psychiatry. 2014.
Abstract
Background: Genetic studies have implicated disrupted-in-schizophrenia-1 (DISC1) as a risk factor for a wide range of mental conditions, including schizophrenia. Because N-methyl-D-aspartate receptor (NMDAR) dysfunction has been strongly linked to the pathophysiology of these conditions, we examined whether the NMDAR is a potential target of DISC1.
Methods: DISC1 was knocked down with a small inference RNA. NMDAR-mediated currents were recorded and NMDAR expression was measured.
Results: We found that cellular knockdown of DISC1 significantly increased NMDAR currents in cortical cultures, which were accompanied by an increase in the expression of NMDAR subunit, GluN2A. NMDAR-mediated synaptic response in prefrontal cortical pyramidal neurons was also increased by DISC1 knockdown in vivo. The effect of DISC1 knockdown on NMDAR currents in cortical cultures was blocked by protein kinase A (PKA) inhibitor, occluded by PKA activator, and prevented by phosphodiesterase 4 inhibitor. Knockdown of DISC1 caused a significant increase of cyclic adenosine monophosphate response element-binding protein (CREB) activity. Inhibiting CREB prevented the DISC1 deficiency-induced increase of NMDAR currents and GluN2A clusters.
Conclusions: Our results suggest that DISC1 exerts an important impact on NMDAR expression and function through a phosphodiesterase 4/PKA/CREB-dependent mechanism, which provides a potential molecular basis for the role of DISC1 in influencing NMDAR-dependent cognitive and emotional processes.
Keywords: CREB; DISC1; GluN2A; NMDA receptors; PKA; schizophrenia.
Copyright © 2014 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
Fig. 1
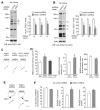
Knockdown of DISC1 increases NMDAR current density and synaptic response in vitro. A, B, Immunoblots and quantification analysis of DISC1 (A: anti-DISC1 440; B: anti-rDISC1 C-term) in rat cortical cultures infected with control shRNA or DISC1 shRNA lentivirus. *: p < 0.01. C, Representative NMDA (100 μM)-elicited current traces in cultured cortical neurons transfected with control shRNA, DISC1 shRNA or DISC1 shRNA plus DISC1-FLR, a full-length DISC1 rescue construct that is insensitive to the DISC1 shRNA. Scale bar: 100 pA, 1 s. D, Cumulative data (mean ± SEM) of NMDAR current density, charge transfer and decay time constant in neuronal cultures with different transfections. *: p < 0.01. #: p < 0.05. E, Representative NMDAR current traces in the absence or presence of ifenprodil (10 μM, a GluN2B blocker) in cultured cortical neurons transfected with control shRNA or DISC1 shRNA. Scale bar: 100 pA, 1 s. F, Cumulative data (mean ± SEM) of GluN2A and GluN2B components in neuronal cultures with different transfections. *: p < 0.01.
Fig. 2
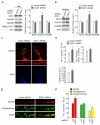
Knockdown of DISC1 increases NMDAR-EPSC in PFC pyramidal neurons in vivo. A, Immunoblots and quantification analysis of DISC1 (detected with anti-DISC1 440) in rat PFC slices taken from animals with stereotaxical injections of control shRNA or DISC1 shRNA lentivirus. #: p < 0.05. B, Dot plots showing the amplitude of NMDAR-EPSC in control shRNA or DISC1 shRNA lentivirus-infected PFC pyramidal neurons. The average data are also shown. *: p < 0.01. Inset: representative NMDAR-EPSC traces. Scale bar: 100 pA, 200 ms. C, Summarized input-output curves of NMDAR-EPSC in pyramidal neurons from rats with the PFC injection of control shRNA or DISC1 shRNA lentivirus. *: p < 0.01, #: p < 0.05. D, Representative NMDAR-EPSC traces in the absence or presence of ifenprodil (10 μM) in PFC pyramidal neurons infected with control shRNA or DISC1 shRNA lentivirus. Scale bar: 50 pA, 100 ms. E, Cumulative data (mean ± SEM) of total NMDAR-EPSC amplitude, GluN2A or GluN2B components, decay time constant and charge transfer in neurons with different viral infections. #: p < 0.05.
Fig. 3
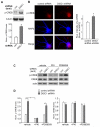
Knockdown of DISC1 increases NMDAR subunit GluN2A expression. A, Immunoblots and quantification analysis of GluN1, GluN2A, GluN2B, GABAAR β2/3 and actin in cultured cortical neurons infected with control shRNA or DISC1 shRNA lentivirus. *: p < 0.01. B, Immunoblots and quantification analysis of surface GluN1, GluN2A, and GluN2B in cultured cortical neurons infected with control shRNA or DISC1 shRNA lentivirus. Actin was used as a control. *: p < 0.05. C, Immunocytochemical images of NMDAR GluN2A subunits and MAP2 in cortical cultures transfected with a control shRNA or DISC1 shRNA. D, Quantitative analysis of GluN2A clusters (density, intensity, size) along the dendrites in control shRNA or DISC1 shRNA-transfected neurons. *: p < 0.01, #: p < 0.05. E, F, Immunocytochemical images (E) and quantitative analysis (F) of synaptic GluN2A clusters (synaptophysin co-localized, yellow puncta) in cortical cultures transfected with control shRNA or DISC1 shRNA. *: p < 0.01.
Fig. 4
PKA activation is required for DISC1 regulation of NMDARs. A, Representative whole-cell NMDAR current traces in cultured cortical neurons transfected with a control shRNA and DISC1 shRNA in the absence or presence of PKI (0.2 μM, a PKA inhibitor), 8-cpt-cAMP (50 μM, a PKA activator), rolipram (0.1 μM, a PDE4 inhibitor), and Bisindolylmaleimide I (Bis I, 0.5 μM, a PKC inhibitor). Scale bar: 100 pA, 1 s. B, Cumulative data (mean ± SEM) of NMDAR current density in transfected neurons with different treatments. *: p < 0.05.
Fig. 5
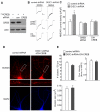
DISC1 knockdown induces an increase of CREB activity. A, Immunoblots and quantification showing the level of p-CREB in cortical cultures infected with control shRNA or DISC1 shRNA lentivirus. Tubulin was used as a control. *: p < 0.01. B, Immunocytochemical images and quantitative analysis of p-CREB in PFC cultures transfected with a control shRNA or DISC1 shRNA. MAP2 was co-stained. *: p < 0.01. C, D, Immunoblots and quantification showing the level of p-CREB and CREB in cortical cultures infected with control shRNA or DISC1 shRNA lentivirus in the presence of vehicle, PKI (0.2 μM) or PD98059 (20 μM, an ERK inhibitor). *: p < 0.001.
Fig. 6
Inhibiting CREB blocks DISC1 regulation of NMDA receptors. A, Western blots in HEK293 cells transfected with FLAG-tagged CREB in the absence or presence of a control siRNA or CREB siRNA. B, Representative whole-cell NMDAR current traces in cultured cortical neurons transfected with a control shRNA or DISC1 shRNA in the absence or presence of CREB siRNA or DN-CREB. Scale bar: 100 pA, 1 s. C, Cumulative data (mean ± SEM) showing NMDAR current density in neurons with different transfections. *: p < 0.05. D, Immunocytochemical images of GluN2A subunits and MAP2 in cortical cultures transfected with a control shRNA or co-transfected DN-CREB with DISC1 shRNA. E, Quantitative analysis of GluN2A clusters (density, intensity, size) along the dendrites in transfected neurons.
Comment in
- DISC1 dynamically regulates synaptic N-methyl-D-aspartate responses in excitatory neurons.
Wang G, Zhu JJ. Wang G, et al. Biol Psychiatry. 2014 Mar 1;75(5):348-50. doi: 10.1016/j.biopsych.2013.12.003. Epub 2013 Dec 16. Biol Psychiatry. 2014. PMID: 24507569 No abstract available.
Similar articles
- Role of DISC1 in Neuronal Trafficking and its Implication in Neuropsychiatric Manifestation and Neurotherapeutics.
Tomoda T, Hikida T, Sakurai T. Tomoda T, et al. Neurotherapeutics. 2017 Jul;14(3):623-629. doi: 10.1007/s13311-017-0556-5. Neurotherapeutics. 2017. PMID: 28664299 Free PMC article. Review. - Knockdown of the aryl hydrocarbon receptor attenuates excitotoxicity and enhances NMDA-induced BDNF expression in cortical neurons.
Lin CH, Chen CC, Chou CM, Wang CY, Hung CC, Chen JY, Chang HW, Chen YC, Yeh GC, Lee YH. Lin CH, et al. J Neurochem. 2009 Nov;111(3):777-89. doi: 10.1111/j.1471-4159.2009.06364.x. Epub 2009 Aug 27. J Neurochem. 2009. PMID: 19712055 - DISC1 regulates N-methyl-D-aspartate receptor dynamics: abnormalities induced by a Disc1 mutation modelling a translocation linked to major mental illness.
Malavasi ELV, Economides KD, Grünewald E, Makedonopoulou P, Gautier P, Mackie S, Murphy LC, Murdoch H, Crummie D, Ogawa F, McCartney DL, O'Sullivan ST, Burr K, Torrance HS, Phillips J, Bonneau M, Anderson SM, Perry P, Pearson M, Constantinides C, Davidson-Smith H, Kabiri M, Duff B, Johnstone M, Polites HG, Lawrie SM, Blackwood DH, Semple CA, Evans KL, Didier M, Chandran S, McIntosh AM, Price DJ, Houslay MD, Porteous DJ, Millar JK. Malavasi ELV, et al. Transl Psychiatry. 2018 Sep 6;8(1):184. doi: 10.1038/s41398-018-0228-1. Transl Psychiatry. 2018. PMID: 30190480 Free PMC article. - Developmental decrease in NMDA receptor desensitization associated with shift to synapse and interaction with postsynaptic density-95.
Li B, Otsu Y, Murphy TH, Raymond LA. Li B, et al. J Neurosci. 2003 Dec 3;23(35):11244-54. doi: 10.1523/JNEUROSCI.23-35-11244.2003. J Neurosci. 2003. PMID: 14657184 Free PMC article. - Mechanisms underlying the role of DISC1 in synaptic plasticity.
Tropea D, Hardingham N, Millar K, Fox K. Tropea D, et al. J Physiol. 2018 Jul;596(14):2747-2771. doi: 10.1113/JP274330. J Physiol. 2018. PMID: 30008190 Free PMC article. Review.
Cited by
- Disrupted in schizophrenia 1 (DISC1) L100P mutants have impaired activity-dependent plasticity in vivo and in vitro.
Tropea D, Molinos I, Petit E, Bellini S, Nagakura I, O'Tuathaigh C, Schorova L, Mitchell KJ, Waddington J, Sur M, Gill M, Corvin AP. Tropea D, et al. Transl Psychiatry. 2016 Jan 12;6(1):e712. doi: 10.1038/tp.2015.206. Transl Psychiatry. 2016. PMID: 26756905 Free PMC article. - Role of DISC1 in Neuronal Trafficking and its Implication in Neuropsychiatric Manifestation and Neurotherapeutics.
Tomoda T, Hikida T, Sakurai T. Tomoda T, et al. Neurotherapeutics. 2017 Jul;14(3):623-629. doi: 10.1007/s13311-017-0556-5. Neurotherapeutics. 2017. PMID: 28664299 Free PMC article. Review. - Histone Modification of Nedd4 Ubiquitin Ligase Controls the Loss of AMPA Receptors and Cognitive Impairment Induced by Repeated Stress.
Wei J, Xiong Z, Lee JB, Cheng J, Duffney LJ, Matas E, Yan Z. Wei J, et al. J Neurosci. 2016 Feb 17;36(7):2119-30. doi: 10.1523/JNEUROSCI.3056-15.2016. J Neurosci. 2016. PMID: 26888924 Free PMC article. - Estradiol reverses excitatory synapse loss in a cellular model of neuropsychiatric disorders.
Erli F, Palmos AB, Raval P, Mukherjee J, Sellers KJ, Gatford NJF, Moss SJ, Brandon NJ, Penzes P, Srivastava DP. Erli F, et al. Transl Psychiatry. 2020 Jan 21;10(1):16. doi: 10.1038/s41398-020-0682-4. Transl Psychiatry. 2020. PMID: 32066698 Free PMC article. - GluD1 knockout mice with a pure C57BL/6N background show impaired fear memory, social interaction, and enhanced depressive-like behavior.
Nakamoto C, Kawamura M, Nakatsukasa E, Natsume R, Takao K, Watanabe M, Abe M, Takeuchi T, Sakimura K. Nakamoto C, et al. PLoS One. 2020 Feb 20;15(2):e0229288. doi: 10.1371/journal.pone.0229288. eCollection 2020. PLoS One. 2020. PMID: 32078638 Free PMC article.
References
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, Clair DM, Muir WJ, Blackwood DH, Porteous DJ. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–23. - PubMed
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. - PubMed
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andradé M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- R01 MH085774/MH/NIMH NIH HHS/United States
- MH-069853/MH/NIMH NIH HHS/United States
- R01 MH069853/MH/NIMH NIH HHS/United States
- R01 MH084233/MH/NIMH NIH HHS/United States
- MH-101690/MH/NIMH NIH HHS/United States
- R21 MH101690/MH/NIMH NIH HHS/United States
- MH-084233/MH/NIMH NIH HHS/United States
- MH-094268/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources