Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans - PubMed (original) (raw)
Review
Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans
Filipe Cabreiro et al. EMBO Mol Med. 2013 Sep.
Abstract
Many animal species live in close association with commensal and symbiotic microbes (microbiota). Recent studies have revealed that the status of gastrointestinal tract microbiota can influence nutrition-related syndromes such as obesity and type-2 diabetes, and perhaps aging. These morbidities have a profound impact in terms of individual suffering, and are an increasing economic burden to modern societies. Several theories have been proposed for the influence of microbiota on host metabolism, but these largely remain to be proven. In this article we discuss how microbiota may be manipulated (via pharmacology, diet, or gene manipulation) in order to alter metabolism, immunity, health and aging in the host. The nematode Caenorhabditis elegans in combination with one microbial species is an excellent, defined model system to investigate the mechanisms of host-microbiota interactions, particularly given the combined power of worm and microbial genetics. We also discuss the multifaceted nature of the worm-microbe relationship, which likely encompasses predation, commensalism, pathogenicity and necromeny.
Keywords: C. elegans; aging; metformin; microbiota; type-2 diabetes.
© 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures
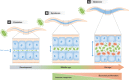
Figure 1. From hunter to prey: the changing relationship between C. elegans and E. coli
Different stages in the course of the life of the worm can serve as models to study how humans interact with their microbiota.
- During development, bacteria mainly serve as a source of food (green), since they are almost entirely crushed by the pharyngeal grinder and live colonies are absent from the lumen of the gut (Kurz et al, 2003).
- In young adults, bacteria that have escaped the action of the grinder proliferate and establish a community within some sections of the lumen of the gut (Portal-Celhay et al, 2012), and exist as commensals or symbionts (green).
- As the worm ages, bacteria proliferating within the lumen of the gut become detrimental to the host (McGee et al, 2011) and can cause severe constipation (Garigan et al, 2002). A possibility is that this reflects changes in bacterial metabolism engendering dysbiosis (red). In addition, blockage of the lumen (Garigan et al, 2002), and changes in bacterial metabolism may be detrimental too (Portal-Celhay et al, 2012). Treatments that block bacterial proliferation extend lifespan, likely by preventing microbial dysbiosis (Garigan et al, ; Gems & Riddle, 2000).
Figure 2. Functions of the microbiota in human and nematode hosts
- Schematic summary of the effects of intestinal microbiota on host fitness. These include protective, structural and metabolic effects.
- The host confers benefits to the microbiota. In the case of C. elegans, the benefits to the microbiota, as hypothesized here, involve cannibalistic commensalism and necromeny.
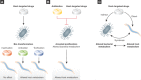
Figure 3. How to test host-targeted drugs in worms
Bacterial roles in drug action on host physiology.
- Biotransformation (activation, inactivation, toxification) by different bacterial strains can alter the efficacy of a host-targeted drug with consequences to host physiology.
- Impact of host-targeted drugs and antibiotics on bacterial metabolism. Antibiotic and host-targeted drug action in bacterial proliferation and/or metabolism influences host physiology either through reduced pathogenesis or the alteration of bacterially derived metabolites.
- Host-targeted drugs can alter the physiology of the worm-bug. Effects of drugs on microbe and/or host can positively or negatively modulate the symbiotic relationship established between host and bacteria. For example, metformin action on E. coli can extend worm lifespan, but acting directly on worms metformin shortens lifespan (Cabreiro et al, 2013).
Similar articles
- Host-microbiota interactions in Caenorhabditis elegans and their significance.
Shapira M. Shapira M. Curr Opin Microbiol. 2017 Aug;38:142-147. doi: 10.1016/j.mib.2017.05.012. Epub 2017 Jun 14. Curr Opin Microbiol. 2017. PMID: 28623729 Review. - Giardia duodenalis-induced alterations of commensal bacteria kill Caenorhabditis elegans: a new model to study microbial-microbial interactions in the gut.
Gerbaba TK, Gupta P, Rioux K, Hansen D, Buret AG. Gerbaba TK, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Mar 15;308(6):G550-61. doi: 10.1152/ajpgi.00335.2014. Epub 2015 Jan 8. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25573177 Free PMC article. - Caenorhabditis elegans: a model to understand host-microbe interactions.
Kumar A, Baruah A, Tomioka M, Iino Y, Kalita MC, Khan M. Kumar A, et al. Cell Mol Life Sci. 2020 Apr;77(7):1229-1249. doi: 10.1007/s00018-019-03319-7. Epub 2019 Oct 4. Cell Mol Life Sci. 2020. PMID: 31584128 Free PMC article. Review. - Genetic and molecular analysis of nematode-microbe interactions.
Tan MW, Shapira M. Tan MW, et al. Cell Microbiol. 2011 Apr;13(4):497-507. doi: 10.1111/j.1462-5822.2011.01570.x. Epub 2011 Jan 30. Cell Microbiol. 2011. PMID: 21276170 Review. - Caenorhabditis elegans as a Convenient Animal Model for Microbiome Studies.
Wu CY, Davis S, Saudagar N, Shah S, Zhao W, Stern A, Martel J, Ojcius D, Yang HC. Wu CY, et al. Int J Mol Sci. 2024 Jun 18;25(12):6670. doi: 10.3390/ijms25126670. Int J Mol Sci. 2024. PMID: 38928375 Free PMC article. Review.
Cited by
- The microbiome: an emerging key player in aging and longevity.
Kim M, Benayoun BA. Kim M, et al. Transl Med Aging. 2020;4:103-116. Epub 2020 Jul 21. Transl Med Aging. 2020. PMID: 32832742 Free PMC article. - Take a Walk to the Wild Side of _Caenorhabditis elegans_-Pathogen Interactions.
Radeke LJ, Herman MA. Radeke LJ, et al. Microbiol Mol Biol Rev. 2021 Mar 17;85(2):e00146-20. doi: 10.1128/MMBR.00146-20. Print 2021 May 19. Microbiol Mol Biol Rev. 2021. PMID: 33731489 Free PMC article. Review. - Autofluorescence as a noninvasive biomarker of senescence and advanced glycation end products in Caenorhabditis elegans.
Komura T, Yamanaka M, Nishimura K, Hara K, Nishikawa Y. Komura T, et al. NPJ Aging Mech Dis. 2021 Jun 7;7(1):12. doi: 10.1038/s41514-021-00061-y. NPJ Aging Mech Dis. 2021. PMID: 34099724 Free PMC article. - Detecting Changes in the Caenorhabditis elegans Intestinal Environment Using an Engineered Bacterial Biosensor.
Rutter JW, Ozdemir T, Galimov ER, Quintaneiro LM, Rosa L, Thomas GM, Cabreiro F, Barnes CP. Rutter JW, et al. ACS Synth Biol. 2019 Dec 20;8(12):2620-2628. doi: 10.1021/acssynbio.9b00166. Epub 2019 Nov 8. ACS Synth Biol. 2019. PMID: 31657907 Free PMC article. - Phytobacter diazotrophicus from Intestine of Caenorhabditis elegans Confers Colonization-Resistance against Bacillus nematocida Using Flagellin (FliC) as an Inhibition Factor.
Niu Q, Liu S, Yin M, Lei S, Rezzonico F, Zhang L. Niu Q, et al. Pathogens. 2022 Jan 10;11(1):82. doi: 10.3390/pathogens11010082. Pathogens. 2022. PMID: 35056030 Free PMC article.
References
- Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Yurova MV, Kovalenko IG, Poroshina TE, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. - PubMed
- Baeriswyl S, Diard M, Mosser T, Leroy M, Maniere X, Taddei F, Matic I. Modulation of aging profiles in isogenic populations of Caenorhabditis elegans by bacteria causing different extrinsic mortality rates. Biogerontology. 2010;11:53–65. - PubMed
- Bailey CJ, Wilcock C, Scarpello JH. Metformin and the intestine. Diabetologia. 2008;51:1552–1553. - PubMed
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources