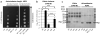PAAR-repeat proteins sharpen and diversify the type VI secretion system spike - PubMed (original) (raw)
. 2013 Aug 15;500(7462):350-353.
doi: 10.1038/nature12453. Epub 2013 Aug 7.
Affiliations
- PMID: 23925114
- PMCID: PMC3792578
- DOI: 10.1038/nature12453
PAAR-repeat proteins sharpen and diversify the type VI secretion system spike
Mikhail M Shneider et al. Nature. 2013.
Abstract
The bacterial type VI secretion system (T6SS) is a large multicomponent, dynamic macromolecular machine that has an important role in the ecology of many Gram-negative bacteria. T6SS is responsible for translocation of a wide range of toxic effector molecules, allowing predatory cells to kill both prokaryotic as well as eukaryotic prey cells. The T6SS organelle is functionally analogous to contractile tails of bacteriophages and is thought to attack cells by initially penetrating them with a trimeric protein complex called the VgrG spike. Neither the exact protein composition of the T6SS organelle nor the mechanisms of effector selection and delivery are known. Here we report that proteins from the PAAR (proline-alanine-alanine-arginine) repeat superfamily form a sharp conical extension on the VgrG spike, which is further involved in attaching effector domains to the spike. The crystal structures of two PAAR-repeat proteins bound to VgrG-like partners show that these proteins sharpen the tip of the T6SS spike complex. We demonstrate that PAAR proteins are essential for T6SS-mediated secretion and target cell killing by Vibrio cholerae and Acinetobacter baylyi. Our results indicate a new model of the T6SS organelle in which the VgrG-PAAR spike complex is decorated with multiple effectors that are delivered simultaneously into target cells in a single contraction-driven translocation event.
Figures
Figure 1
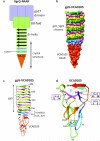
Crystal structure of the VCA0105 PAAR-repeat protein bound to its VgrG-like partner. a, Schematic representation of the conserved domains comprising the VgrG-PAAR complex. The last strands of the β-helix that form the PAAR binding site are in light blue. Gray arrow shows the fragment roughly corresponding to the crystal structure. b, Molecular surface representation of the gp5_VCA0018-VCA0105 complex crystal structure. Each protein chain is labeled with its own color. c, Ribbon diagram of the gp5_VCA0018- VCA0105 complex. d, The polypeptide chain of the VCA0105 PAAR protein is colored in rainbow colors with N terminus in blue and C terminus in red. Residues responsible for Zn binding are labeled.
Figure 2
PAAR proteins are required for full functionality of the T6SS in Vibrio cholerae and Acinetobacter baylyi. a, Recovery of viable E. coli MG1655 after co-incubation with A. baylyi ADP1 (WT) and its T6SS and PAAR genes knockout mutants. The following genes were inactivated in the mutants shown: T6S¯ – aciad2688 to aciad2694; 2681¯ – aciad2681; 0051-52¯ – both aciad0051 and aciad0052; 3×P¯ – all three PAAR genes aciad0051, aciad0052 and aciad2681. Right subpanel: Leaky, basal expression of the aciad2681 PAAR gene from plasmid pMMB67EH, labeled as p2681, restores the killing defect in the triple PAAR mutant. b, Recovery of E. coli MG1655 colony forming units after co-incubation with V. cholerae 2740-80 and its T6SS and PAAR genes knockout mutants, which are labeled as follows: T6S¯ – vipA; 105¯ – vca0105; 284¯ – vca0284; 2×P¯ – vca0105 and vca0284. Symbols *, **, and *** indicate deviations from the WT with p-values of 6×10−3, 8×10−3, and 5×10−7, respectively, for a sample size of 8. Error bars represent one standard deviation. c, SDS-PAGE assay for T6SS-dependent secretion of Hcp proteins by the parental strains and T6SS and PAAR genes knockout mutants. Panels a and c show one out of three experiments with similar outcomes.
Figure 3
The vsvG epitope-tagged PAAR protein ACIAD2681 is secreted by A. baylyi ADP1. Left panels: T6SS-dependent secretion of vsvG epitope-tagged ACIAD2681 expressed from plasmid pMMB67EH. Right panel: vsvG epitope-tagged ACIAD2681 fully restores the Hcp secretion defect of the triple knockout PAAR mutant of A. baylyi ADP1. The mutants are labeled as in Fig. 2. A representative of three identical experiments was chosen for each panel.
Figure 4
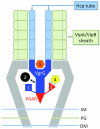
Multiple Effector TRanslocation VgrG (MERV) model for the organization of the T6SS central spike/baseplate. Effectors are predicted to be loaded onto the spike complex by five distinct mechanisms: 1) C-terminal extensions of the VgrG spike; 2) Non-covalent binding to the VgrG spike; 3) N- or C-terminal extensions of the PAAR protein; 4) Non- covalent binding to the PAAR protein or its extension domains; 5) Incorporation into the cavity formed by the gp27 domain of VgrG. A single T6SS sheath contraction event translocates the VgrG spike with all of its cargo proteins into a nearby target cell. Other proteins making up the T6SS “baseplate” are not labeled but presumably reside within or attached to the inner and outer membranes and peptidoglycan layer (IM, OM, and PG, respectively).
Comment in
- Microbiology: a weapon for bacterial warfare.
Filloux A. Filloux A. Nature. 2013 Aug 15;500(7462):284-5. doi: 10.1038/nature12545. Epub 2013 Aug 7. Nature. 2013. PMID: 23925115 No abstract available.
Similar articles
- VgrG Spike Dictates PAAR Requirement for the Assembly of the Type VI Secretion System.
Liang X, Zheng HY, Zhao YJ, Zhang YQ, Pei TT, Cui Y, Tang MX, Xu P, Dong T. Liang X, et al. J Bacteriol. 2023 Feb 22;205(2):e0035622. doi: 10.1128/jb.00356-22. Epub 2023 Jan 19. J Bacteriol. 2023. PMID: 36655996 Free PMC article. - VgrG and PAAR Proteins Define Distinct Versions of a Functional Type VI Secretion System.
Cianfanelli FR, Alcoforado Diniz J, Guo M, De Cesare V, Trost M, Coulthurst SJ. Cianfanelli FR, et al. PLoS Pathog. 2016 Jun 28;12(6):e1005735. doi: 10.1371/journal.ppat.1005735. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27352036 Free PMC article. - Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair.
Altindis E, Dong T, Catalano C, Mekalanos J. Altindis E, et al. mBio. 2015 Mar 10;6(2):e00075. doi: 10.1128/mBio.00075-15. mBio. 2015. PMID: 25759499 Free PMC article. - VgrG, Tae, Tle, and beyond: the versatile arsenal of Type VI secretion effectors.
Durand E, Cambillau C, Cascales E, Journet L. Durand E, et al. Trends Microbiol. 2014 Sep;22(9):498-507. doi: 10.1016/j.tim.2014.06.004. Epub 2014 Jul 17. Trends Microbiol. 2014. PMID: 25042941 Review. - A view to a kill: the bacterial type VI secretion system.
Ho BT, Dong TG, Mekalanos JJ. Ho BT, et al. Cell Host Microbe. 2014 Jan 15;15(1):9-21. doi: 10.1016/j.chom.2013.11.008. Epub 2013 Dec 11. Cell Host Microbe. 2014. PMID: 24332978 Free PMC article. Review.
Cited by
- Intraspecies Competition in Serratia marcescens Is Mediated by Type VI-Secreted Rhs Effectors and a Conserved Effector-Associated Accessory Protein.
Alcoforado Diniz J, Coulthurst SJ. Alcoforado Diniz J, et al. J Bacteriol. 2015 Jul;197(14):2350-60. doi: 10.1128/JB.00199-15. Epub 2015 May 4. J Bacteriol. 2015. PMID: 25939831 Free PMC article. - Vibrio vulnificus Type 6 Secretion System 1 Contains Anti-Bacterial Properties.
Church SR, Lux T, Baker-Austin C, Buddington SP, Michell SL. Church SR, et al. PLoS One. 2016 Oct 31;11(10):e0165500. doi: 10.1371/journal.pone.0165500. eCollection 2016. PLoS One. 2016. PMID: 27798649 Free PMC article. - Structure of the T4 baseplate and its function in triggering sheath contraction.
Taylor NM, Prokhorov NS, Guerrero-Ferreira RC, Shneider MM, Browning C, Goldie KN, Stahlberg H, Leiman PG. Taylor NM, et al. Nature. 2016 May 19;533(7603):346-52. doi: 10.1038/nature17971. Nature. 2016. PMID: 27193680 - Interbacterial competition and anti-predatory behaviour of environmental Vibrio cholerae strains.
Drebes Dörr NC, Blokesch M. Drebes Dörr NC, et al. Environ Microbiol. 2020 Oct;22(10):4485-4504. doi: 10.1111/1462-2920.15224. Epub 2020 Oct 2. Environ Microbiol. 2020. PMID: 32885535 Free PMC article. - Polymorphic Toxins and Their Immunity Proteins: Diversity, Evolution, and Mechanisms of Delivery.
Ruhe ZC, Low DA, Hayes CS. Ruhe ZC, et al. Annu Rev Microbiol. 2020 Sep 8;74:497-520. doi: 10.1146/annurev-micro-020518-115638. Epub 2020 Jul 17. Annu Rev Microbiol. 2020. PMID: 32680451 Free PMC article. Review.
References
- Kapitein N, Mogk A. Deadly syringes: type VI secretion system activities in pathogenicity and interbacterial competition. Curr Opin Microbiol. 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI026289/AI/NIAID NIH HHS/United States
- R37 AI018045/AI/NIAID NIH HHS/United States
- R01 AI018045/AI/NIAID NIH HHS/United States
- AI-026289/AI/NIAID NIH HHS/United States
- AI-01845/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases