Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity - PubMed (original) (raw)
. 2013 Sep;123(9):3941-51.
doi: 10.1172/JCI66413. Epub 2013 Aug 8.
Jialie Luo, Aihua Qian, Junhui Du, Qing Yang, Shentai Zhou, Weihua Yu, Guangwei Du, Richard B Clark, Edgar T Walters, Susan M Carlton, Hongzhen Hu
Affiliations
- PMID: 23925292
- PMCID: PMC3754244
- DOI: 10.1172/JCI66413
Retinoids activate the irritant receptor TRPV1 and produce sensory hypersensitivity
Shijin Yin et al. J Clin Invest. 2013 Sep.
Abstract
Retinoids are structurally related derivatives of vitamin A and are required for normal vision as well as cell proliferation and differentiation. Clinically, retinoids are effective in treating many skin disorders and cancers. Application of retinoids evokes substantial irritating side effects, including pain and inflammation; however, the precise mechanisms accounting for the sensory hypersensitivity are not understood. Here we show that both naturally occurring and synthetic retinoids activate recombinant or native transient receptor potential channel vanilloid subtype 1 (TRPV1), an irritant receptor for capsaicin, the pungent ingredient of chili peppers. In vivo, retinoids produced pain-related behaviors that were either eliminated or significantly reduced by genetic or pharmacological inhibition of TRPV1 function. These findings identify TRPV1 as an ionotropic receptor for retinoids and provide cellular and molecular insights into retinoid-evoked hypersensitivity. These findings also suggest that selective TRPV1 antagonists are potential therapeutic drugs for treating retinoid-induced sensory hypersensitivity.
Figures
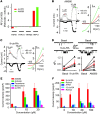
Figure 1. Activation of recombinant TRPV1 by both synthetic and naturally occurring retinoids.
(A) AM580 (100 μM) and 4-HPR (100 μM) specifically evoked [Ca2+]i responses in HEK293T cells transfected with recombinant TRPV1. Neither retinoid had an effect on HEK293T cells transfected with TRPA1, TRPV3, or TRPM8. The y axis refers to a net increase of relative fluorescence units (RFU) induced by AM580 and 4-HPR after subtraction of the baseline response to vehicle alone. (B) Left: representative traces showing that AM580-activated TRPV1-mediated current in a concentration-dependent manner. Right: representative current–voltage (I–V) curves taken at specified time points from the traces on the left illustrate that an outwardly rectifying whole-cell current was evoked by AM580 at 1 μM (b), whereas 10 μM (c) AM580 activated a current with a linear I-V relationship in a TRPV1-expressing HEK293T cell (a refers to the baseline response). (C) An endogenous retinoid, 9-cis-RA, also activated TRPV1 current in a concentration-dependent manner similar to that activated by AM580 (a refers to the baseline response; b and c indicate responses activated by 10 and 30 μM 9-cis-RA, respectively). (D) Both AM580 (10 μM, n = 5) and 9-cis-RA (10 μM, n = 4) increased single channel open probability (nPo) in inside-out patches excised from TRPV1-expressing HEK293T cells (*P < 0.05). (E) Synthetic retinoids activate TRPV1 in a concentration-dependent manner. (F) Summary of effects of selected endogenous retinoids on TRPV1-expressing cells. Currents were recorded at a membrane potential of –60 mV. n = 5–10 per concentration for all retinoids examined.
Figure 2. Pharmacological inhibition or genetic ablation of TRPV1 function abolished AM580-evoked membrane current, membrane depolarization, and action potential firing in DRG neurons.
(A) Representative current traces show that AM580 (30 μM) activated an outward current at +60 mV and an inward current at –60 mV in a wild-type DRG neuron. The whole-cell currents evoked by AM580 were substantially suppressed by the selective TRPV1 antagonist AMG9810 (0.1 μM). The inhibitory effect of AMG9810 was partially reversible after washout. (B) Current-voltage relationship of AM580-activated current taken at time points specified in A. (C) Quantification of current responses to AM580 in DRG neurons voltage-clamped at –60 mV. AMG9810 (0.1 μM) significantly inhibited the AM580 response (*P < 0.05, n = 6). (D) Representative voltage traces illustrate that AM580 evoked a membrane depolarization and increased firing rate of a capsaicin-sensitive, tonically firing wild-type DRG neuron. (E) AM580 produced membrane depolarization and action potential firing in an electrically silent DRG neuron from a wild-type mouse that was also sensitive to both AITC and capsaicin. (F) AITC but not AM580 or capsaicin induced a membrane depolarization in a DRG neuron from a Trpv1–/– mouse. Drug concentrations: AM580, 30 μM; capsaicin, 1 μM; and AITC, 100 μM. MP, membrane potential.
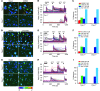
Figure 3. TRPV1 is the sole target of retinoids in sensory nociceptors.
AM580 (A–C), 9-cis-RA (D–F), ATRA (G–I), and capsaicin evoked [Ca2+]i responses in a subset of DRG neurons from Trpv1+/+ but not _Trpv1–/–_mice. (A, D, and G) Representative Fura-2 ratiometric images of cultured DRG neurons show that AM580 (A), 9-cis-RA (D), and ATRA (G) evoked [Ca2+]i responses in a subset of DRG neurons from Trpv1+/+ but not Trpv1–/– mice. The color of the neurons switching from blue to green or red indicates the increase of [Ca2+]i. (B, E, and H) Representative traces illustrate that AM580 (B), 9-cis-RA (E), ATRA (H), or capsaicin elicited [Ca2+]i responses in Trpv1+/+ but not Trpv1–/– DRG neurons. AITC evoked similar [Ca2+]i responses in both Trpv1+/+ and Trpv1–/– DRG neurons. Each trace corresponds to the change of fluorescence ratio in a single neuron. Neurons were exposed to each retinoid (5 μM AM580, 30 μM 9-cis-RA, or 300 μM ATRA), 0.3 μM capsaicin, 100 μM AITC, and 100 mM KCl for the indicated times. (C, F, and I) Percentage of DRG neurons responding to AM580 (C), 9-cis-RA (F), ATRA (I), capsaicin, AITC, and KCl in neurons isolated from Trpv1+/+ or Trpv1–/– mice (n ≥ 330 per genotype for AM580; n 350 per genotype for 9-cis-RA and ATRA).
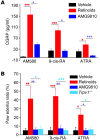
Figure 4. TRPV1 mediates retinoid-evoked CGRP release and paw edema.
(A) AM580 (30 μM), 9-cis-RA (300 μM), and ATRA (1000 μM) increased CGRP levels in the perfusates from rat colon segments. AMG9810 (1 μM) significantly reduced the effect of all retinoids tested (n = 6). *P < 0.05 and ***P < 0.001 versus vehicle; +P < 0.05 and +++P < 0.001 versus AMG9810. (B) Intraplantar injections of 20 μl of each AM580 (100 nmol), 9-cis-RA (100 nmol), or ATRA (600 nmol) significantly increased paw volume compared with that injected with vehicle controls. The paw edema ratio is the percentage increase of paw volume induced by retinoids. The effects of retinoids were markedly attenuated by AMG9810 (30 mg/kg, i.p. injection) or abolished in the Trpv1–/– mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle; +P < 0.05, ++P < 0.01 versus AMG9810; #P < 0.05, ###P < 0.001 versus Trpv1–/–. n = 6–10 animals per condition.
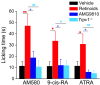
Figure 5. Ablation of retinoid-induced nocifensive responses by genetic deletion or pharmacological blockade of TRPV1.
Intraplantar injection of 20 μl of each AM580 (100 nmol), 9-cis-RA (100 nmol), or ATRA (600 nmol) produced flinching and licking behaviors that were significantly reduced by i.p. injection of AMG9810 (50 mg/kg) 30 minutes before paw injection of retinoids. Genetic ablation of TRPV1 function totally abolished the nocifensive responses evoked by retinoids. *P < 0.05, **P < 0.01 versus vehicle; +P < 0.05 versus AMG9810; and #P < 0.05, ##P < 0.01 versus Trpv1–/–. n = 6–10 animals per condition.
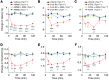
Figure 6. Pharmacological or genetic ablation of TRPV1 function abolishes retinoid-induced sensory hypersensitivity.
(A–C) Time course of thermal hypersensitivity in animals treated with AM580 (A), 9-cis-RA (B). or ATRA (C). Intraplantar injection of 10 μl of each retinoid (AM580, 2 nmol; 9-cis-RA, 3 nmol; and ATRA, 30 nmol; red traces) induced thermal hyperalgesia in Trpv1+/+ mice. AMG9810 (10 mg/kg; i.p. injection;green traces) abolished the effect of selected retinoids. Retinoid-elicited thermal hypersensitivity was also abolished in Trpv1–/– mice (blue traces). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle; ++P < 0.01, +++P < 0.001 versus AMG9810; ##P < 0.01, ###P < 0.001 versus Trpv1–/–. (D–F) Time course of mechanical allodynia in animals treated with AM580 (D), 9-cis-RA (E), and ATRA (F). Intraplantar injection of 10 μl of each retinoid (AM580, 2 nmol; 9-cis-RA, 3 nmol; and ATRA, 30 nmol; red traces) produced mechanical hypersensitivity in Trpv1+/+ mice, which was abolished by i.p. injection of AMG9810 (10 mg/kg; green traces). Retinoid-elicited mechanical hypersensitivity was also abolished in the Trpv1–/– mice (blue traces). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle; +P < 0.05, ++P < 0.01, +++P < 0.001 versus AMG9810; and #P < 0.05, #P < 0.01 versus Trpv1–/–. Please note that no effect was observed upon injection of 10 μl vehicle alone (0.9% saline; black traces). n = 5–10 animals per condition. Baseline values for mechanical and thermal testing are listed in Supplemental Table 2.
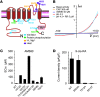
Figure 7. The vanilloid-binding pocket of the TRPV1 confers retinoid sensitivity.
(A) Schematic diagram illustrates structural elements required for activation/modulation of TRPV1 by capsaicin (blue circle), protein phosphorylation (green circle), protons (yellow circle), and heat (purple circle). (B) Representative I-V curves illustrate that the chicken TRPV1 was activated by pH 4.3 but not AM580 or capsaicin (n = 5). The nonselective TRP channel blocker ruthenium red (RR) abolished the inward but not the outward proton-activated current. (C) Quantification of EC50 values for AM580-activated currents at –60 mV in wild-type or TRPV1 mutants with disrupted vanilloid-binding pocket. (D) 9-cis-RA–activated (30 μM) membrane currents (at –60 mV) were nearly abolished in TRPV1 Y512A or S513Y mutant but not S503A mutant (n = 4–7 per condition).
Similar articles
- Persistent Nociception Triggered by Nerve Growth Factor (NGF) Is Mediated by TRPV1 and Oxidative Mechanisms.
Eskander MA, Ruparel S, Green DP, Chen PB, Por ED, Jeske NA, Gao X, Flores ER, Hargreaves KM. Eskander MA, et al. J Neurosci. 2015 Jun 3;35(22):8593-603. doi: 10.1523/JNEUROSCI.3993-14.2015. J Neurosci. 2015. PMID: 26041925 Free PMC article. - LE135, a retinoid acid receptor antagonist, produces pain through direct activation of TRP channels.
Yin S, Luo J, Qian A, Yu W, Hu H. Yin S, et al. Br J Pharmacol. 2014 Mar;171(6):1510-20. doi: 10.1111/bph.12543. Br J Pharmacol. 2014. PMID: 24308840 Free PMC article. - Oxytocin Modulates Nociception as an Agonist of Pain-Sensing TRPV1.
Nersesyan Y, Demirkhanyan L, Cabezas-Bratesco D, Oakes V, Kusuda R, Dawson T, Sun X, Cao C, Cohen AM, Chelluboina B, Veeravalli KK, Zimmermann K, Domene C, Brauchi S, Zakharian E. Nersesyan Y, et al. Cell Rep. 2017 Nov 7;21(6):1681-1691. doi: 10.1016/j.celrep.2017.10.063. Cell Rep. 2017. PMID: 29117570 Free PMC article. - Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review. - Capsaicin, Nociception and Pain.
Frias B, Merighi A. Frias B, et al. Molecules. 2016 Jun 18;21(6):797. doi: 10.3390/molecules21060797. Molecules. 2016. PMID: 27322240 Free PMC article. Review.
Cited by
- TRP Channels as Drug Targets to Relieve Itch.
Xie Z, Hu H. Xie Z, et al. Pharmaceuticals (Basel). 2018 Oct 6;11(4):100. doi: 10.3390/ph11040100. Pharmaceuticals (Basel). 2018. PMID: 30301231 Free PMC article. Review. - Molecular and cellular mechanisms that initiate pain and itch.
Luo J, Feng J, Liu S, Walters ET, Hu H. Luo J, et al. Cell Mol Life Sci. 2015 Sep;72(17):3201-23. doi: 10.1007/s00018-015-1904-4. Epub 2015 Apr 18. Cell Mol Life Sci. 2015. PMID: 25894692 Free PMC article. Review. - Estrogen metabolites increase nociceptor hyperactivity in a mouse model of uterine pain.
Xie Z, Feng J, Cai T, McCarthy R, Eschbach MD 2nd, Wang Y, Zhao Y, Yi Z, Zang K, Yuan Y, Hu X, Li F, Liu Q, Das A, England SK, Hu H. Xie Z, et al. JCI Insight. 2022 May 23;7(10):e149107. doi: 10.1172/jci.insight.149107. JCI Insight. 2022. PMID: 35420999 Free PMC article. - Improvement of mild photoaged facial skin in middle-aged Chinese females by a supramolecular retinol plus acetyl hexapeptide-1 containing essence.
Ye Y, Li Y, Xu C, Wei X. Ye Y, et al. Skin Health Dis. 2023 May 7;3(4):e239. doi: 10.1002/ski2.239. eCollection 2023 Aug. Skin Health Dis. 2023. PMID: 37538317 Free PMC article. - Surfactant cocamide monoethanolamide causes eye irritation by activating nociceptor TRPV1 channels.
Zhao F, Wang S, Li Y, Wang J, Wang Y, Zhang C, Li Y, Huang L, Yu Y, Zheng J, Yu B, Pessah IN, Cao Z. Zhao F, et al. Br J Pharmacol. 2021 Sep;178(17):3448-3462. doi: 10.1111/bph.15491. Epub 2021 Jun 1. Br J Pharmacol. 2021. PMID: 33837959 Free PMC article.
References
- Geria AN, Lawson CN, Halder RM. Topical retinoids for pigmented skin. J Drugs Dermatol. 2011;10(5):483–489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases