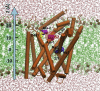Coupled global and local changes direct substrate translocation by neurotransmitter-sodium symporter ortholog LeuT - PubMed (original) (raw)
Coupled global and local changes direct substrate translocation by neurotransmitter-sodium symporter ortholog LeuT
Mary Hongying Cheng et al. Biophys J. 2013.
Abstract
Significant advances have been made in recent years in characterizing neurotransmitter:sodium symporter (NSS) family structure and function. Yet, many time-resolved events and intermediates that control the various stages of transport cycle remain to be elucidated. Whether NSSs harbor one or two sites for binding their substrates (neurotransmitters or amino acids), and what the role of the secondary site S2 is, if any, are still unresolved. Using molecular modeling and simulations for LeuT, a bacterial NSS, we present a comprehensive account of substrate-binding and -stabilization events, and subsequently triggered interactions leading to substrate (alanine) release. LeuT instantaneous conformation as it reconfigures from substrate-receiving (outward-facing) to -releasing (inward-facing) state appears to be a determinant of its affinity to bind substrate at site S2. In the outward-facing state, S1 robustly binds alanine and regulates subsequent redistribution of interactions to trigger extracellular gate closure; whereas S2 is only a transient binding site. The substrate-binding affinity at S2 increases in an intermediate close to inward-facing state. LeuT harbors the two substrate-binding sites, and small displacements of second substrate near S2 are observed to induce concerted small translocations in the substrate bound to primary site S1, although complete release requires collective structural rearrangements that fully expose the intracellular vestibule to the cytoplasm.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
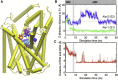
LeuT structure and the locations of the crystallographically observed sodium ions Na1 and Na2, and the substrate-binding sites S1 and S2. A snapshot at t = 2 ns of cMD run 2 is displayed, where the OF_o_ structure (PDB:
3TT1
) was used as input (orange cylinders). POPC molecules are represented by green sticks, their phosphorus atoms in tan spheres; water molecules are shown in pink, and the sodium ions Na1 and Na2, in blue spheres (in all figures). The sites S1 and S2 are indicated by semitransparent spheres. S1 is the primary substrate-binding site observed in the OF_c∗_ crystal structure (PDB:
2A65
); and S2, the more recently proposed secondary site. The figure displays the initial positions of Ala-1 and Ala-2 (purple van der Waals (vdW) spheres), at ∼10 Å and 15 Å along the z axis relative to S1, before MD runs 2–8 (Table 1) for exploring the binding and stabilization of substrate(s) in the OF_o_∗ state. For clarity, TM11 (R446 to E478) is not shown.
Figure 2
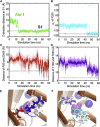
Alanine readily locates and binds either S1 or S2 in the OF open state of LeuT. (A and B) Time evolutions of the instantaneous positions of Ala-1 (green) and Ala-2 (blue), along the z axis normal to membrane (see Fig. 1), with respect to primary site S1. Trajectories refer to the simulations of the transition OF_o_ → OF_o∗_ (runs 2 and 5 in respective panels A and B). The dotted red line indicates the position of site S2 (12) along the z axis; and the dotted gray line, that of S1 (at z = 0). (C and D) Binding of Ala (purple vdW) to the respective sites S2 and S1, observed in the respective runs 2 and 5. The pose in (D) is consistent with that of Leu in the OF_c∗_ crystal structure, isolated from the EC region by the aromatic residues Y108 (violet) and F253 (cyan). EC gate residues R30 (blue), D404 (red), Y108, and F253 are displayed in licorice. Transparent yellow and green regions display the hydrophobic and hydrophilic residues that line the S1 and S2 sites. TM helices 1, 3, 6, and 10 and EC loop EL4b, involved in substrate coordination, are labeled, with suffix a or b indicating the N- or C-terminal segments of the broken helices 1 and 6.
Figure 3
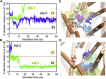
Substrate binding to site S1 prompts the closure of EC gates. Time evolution of the (A) distance of Ala-1 (along the z axis) from S1; (B) χ_1 angle of F253; (C) distance between EC gating residues R30 and D404; and (D) distance between EC gating residues Y108 and F253. (E) Salt bridge formed intermittently between R30 (blue) and D404 (red). Ala-1 is shown in purple vdW spheres. White sticks show the side-chain orientations in the OF_o crystal structure in both E and F. (F) Isomerization of F253 (cyan) brings its aromatic side chain into close proximity of Y108 (violet). Results refer to aMD simulation of substrate binding in the OF state, OF_o_→ OF_c∗_ (run 5). LeuT gradually approaches the OF_c∗_ crystal structure (dashed lines), succeeding the binding of Ala-1 to S1 (around 15 ns; panel A), evidenced by F253 isomerization (B) and ensuing closer association of Y108-F253 (D and F) and R30-D404 salt bridge formation (C and E). Light-color curves in A, C, and D show data collected at 4 ps intervals, and heavy colors, those averaged over 10 such snapshots.
Figure 4
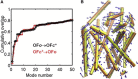
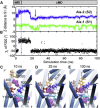
Correlation between theoretically predicted structural changes and those experimentally observed between the open and closed states of OF LeuT (A) Cumulative overlap between ANM modes and experimentally observed structural change from OF_o_ to OF_c∗_ (black), and backward (red); and (B) comparison of the second ANM mode (dark blue arrows) calculated for OF_o_ state (orange), and the structural change between OF_o_ and OF_c∗_ (yellow) structures.
Figure 5
Simultaneous binding of Ala-1 and Ala-2 to S1 and S2 sites is assisted by the R30-D404 salt bridge formation. (A) Equilibrated OF_c∗_ conformer (yellow cylinder) in the presence of two alanines (purple, space-filling) and two sodium ions (blue spheres). EC gate residues R30 (blue), D404 (red), Y108 (violet), and F253 (cyan) are displayed in licorice. (B) Time evolution of the z_-distance of Ala-1 and Ala-2 from S1. Gray dashed lines indicate the positions of S1 and S2. (C) Time evolution of the O-N distance between R30 and D404. Salt bridge formation between these residues helps stabilize the substrate at site S2. Results refer to tMD run 7 (t ≤ 8 ns) for simulating the transition OF_o∗ → OF_c∗_ and aMD run 9 (8 ≤ t ≤ 50 ns) of the equilibrium dynamics at the OF_c∗_ state (see Table 1). Gray vertical bar marks the switch from tMD to aMD.
Figure 6
Stable binding of Ala-2 and its coupling to Ala-1 and F320 side-chain rotation in an intermediate occluded state visited during the global transition OF_c∗→IF_c∗. Results refer to 6.8 ns tMD (run 10) to initiate the transition, followed by 93.2 ns conventional MD (run 11). The gray vertical bar marks the switch from tMD to cMD. Time evolutions of (A) the _z_-axis distance of Ala-1 and Ala-2 mass centers from the S1 site and (B) the _χ_1 dihedral angle of F320. Snapshots at (C) 10 ns, (D) 25 ns, and (E) 100 ns are shown, where the two alanines (purple vdW spheres) are displayed with respect to R30 (blue), D404 (red), Y108 (violet), F253 (cyan), F320 (orange), and L400 (pink). Note the seclusion of Ala-1 by F253-Y108 aromatic side-chains association that serves as an EC gate, and the coordination of Ala-2 by the salt bridge forming residues R30-D404 and the hydrophobic pair F320 and L400.
Similar articles
- Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site.
Piscitelli CL, Krishnamurthy H, Gouaux E. Piscitelli CL, et al. Nature. 2010 Dec 23;468(7327):1129-32. doi: 10.1038/nature09581. Nature. 2010. PMID: 21179170 Free PMC article. - Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue.
Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Zhao Y, et al. Nature. 2011 Jun 2;474(7349):109-13. doi: 10.1038/nature09971. Epub 2011 Apr 24. Nature. 2011. PMID: 21516104 Free PMC article. - Microseconds simulations reveal a new sodium-binding site and the mechanism of sodium-coupled substrate uptake by LeuT.
Zomot E, Gur M, Bahar I. Zomot E, et al. J Biol Chem. 2015 Jan 2;290(1):544-55. doi: 10.1074/jbc.M114.617555. Epub 2014 Nov 7. J Biol Chem. 2015. PMID: 25381247 Free PMC article. - The use of LeuT as a model in elucidating binding sites for substrates and inhibitors in neurotransmitter transporters.
Loland CJ. Loland CJ. Biochim Biophys Acta. 2015 Mar;1850(3):500-10. doi: 10.1016/j.bbagen.2014.04.011. Epub 2014 Apr 24. Biochim Biophys Acta. 2015. PMID: 24769398 Review. - Substrate and drug binding sites in LeuT.
Nyola A, Karpowich NK, Zhen J, Marden J, Reith ME, Wang DN. Nyola A, et al. Curr Opin Struct Biol. 2010 Aug;20(4):415-22. doi: 10.1016/j.sbi.2010.05.007. Epub 2010 Jun 16. Curr Opin Struct Biol. 2010. PMID: 20739005 Free PMC article. Review.
Cited by
- Exploring the conformational transitions of biomolecular systems using a simple two-state anisotropic network model.
Das A, Gur M, Cheng MH, Jo S, Bahar I, Roux B. Das A, et al. PLoS Comput Biol. 2014 Apr 3;10(4):e1003521. doi: 10.1371/journal.pcbi.1003521. eCollection 2014 Apr. PLoS Comput Biol. 2014. PMID: 24699246 Free PMC article. - Properties of an inward-facing state of LeuT: conformational stability and substrate release.
Grouleff J, Søndergaard S, Koldsø H, Schiøtt B. Grouleff J, et al. Biophys J. 2015 Mar 24;108(6):1390-1399. doi: 10.1016/j.bpj.2015.02.010. Biophys J. 2015. PMID: 25809252 Free PMC article. - NbIT--a new information theory-based analysis of allosteric mechanisms reveals residues that underlie function in the leucine transporter LeuT.
LeVine MV, Weinstein H. LeVine MV, et al. PLoS Comput Biol. 2014 May 1;10(5):e1003603. doi: 10.1371/journal.pcbi.1003603. eCollection 2014 May. PLoS Comput Biol. 2014. PMID: 24785005 Free PMC article. - Quantitative Assessment of the Energetics of Dopamine Translocation by Human Dopamine Transporter.
Cheng MH, Kaya C, Bahar I. Cheng MH, et al. J Phys Chem B. 2018 May 31;122(21):5336-5346. doi: 10.1021/acs.jpcb.7b10340. Epub 2017 Dec 26. J Phys Chem B. 2018. PMID: 29232131 Free PMC article. - Shared dynamics of LeuT superfamily members and allosteric differentiation by structural irregularities and multimerization.
Ponzoni L, Zhang S, Cheng MH, Bahar I. Ponzoni L, et al. Philos Trans R Soc Lond B Biol Sci. 2018 Jun 19;373(1749):20170177. doi: 10.1098/rstb.2017.0177. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29735731 Free PMC article.
References
- Reith M., editor. Neurotransmitter Transporters: Structure, Function, and Regulations. Humana; Totowa, NJ: 2002.
- Yamashita A., Singh S.K., Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. - PubMed
- Zomot E., Bakan A., Bahar I. Sodium-coupled Secondary Transporters: Insights from Structure-based Computations. In: Roux B., editor. Molecular Machines. Word Scientific Publishing Co. Pte. Ltd.; Singapore: 2011. pp. 199–229.
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103712/GM/NIGMS NIH HHS/United States
- R01 GM086238/GM/NIGMS NIH HHS/United States
- R01GM086238/GM/NIGMS NIH HHS/United States
- P41GM103712-01/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources