Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma - PubMed (original) (raw)
Clinical Trial
. 2013 Sep 10;31(26):3212-8.
doi: 10.1200/JCO.2012.47.2464. Epub 2013 Aug 12.
Paul Mulholland, Bart Neyns, L Burt Nabors, Mario Campone, Antje Wick, Warren Mason, Tom Mikkelsen, Surasak Phuphanich, Lynn S Ashby, John Degroot, Rao Gattamaneni, Lawrence Cher, Mark Rosenthal, Franz Payer, Juliane M Jürgensmeier, Rakesh K Jain, A Gregory Sorensen, John Xu, Qi Liu, Martin van den Bent
Affiliations
- PMID: 23940216
- PMCID: PMC4021043
- DOI: 10.1200/JCO.2012.47.2464
Clinical Trial
Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma
Tracy T Batchelor et al. J Clin Oncol. 2013.
Abstract
Purpose: A randomized, phase III, placebo-controlled, partially blinded clinical trial (REGAL [Recent in in Glioblastoma Alone and With Lomustine]) was conducted to determine the efficacy of cediranib, an oral pan-vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor, either as monotherapy or in combination with lomustine versus lomustine in patients with recurrent glioblastoma.
Patients and methods: Patients (N = 325) with recurrent glioblastoma who previously received radiation and temozolomide were randomly assigned 2:2:1 to receive (1) cediranib (30 mg) monotherapy; (2) cediranib (20 mg) plus lomustine (110 mg/m(2)); (3) lomustine (110 mg/m(2)) plus a placebo. The primary end point was progression-free survival based on blinded, independent radiographic assessment of postcontrast T1-weighted and noncontrast T2-weighted magnetic resonance imaging (MRI) brain scans.
Results: The primary end point of progression-free survival (PFS) was not significantly different for either cediranib alone (hazard ratio [HR] = 1.05; 95% CI, 0.74 to 1.50; two-sided P = .90) or cediranib in combination with lomustine (HR = 0.76; 95% CI, 0.53 to 1.08; two-sided P = .16) versus lomustine based on independent or local review of postcontrast T1-weighted MRI.
Conclusion: This study did not meet its primary end point of PFS prolongation with cediranib either as monotherapy or in combination with lomustine versus lomustine in patients with recurrent glioblastoma, although cediranib showed evidence of clinical activity on some secondary end points including time to deterioration in neurologic status and corticosteroid-sparing effects.
Trial registration: ClinicalTrials.gov NCT00777153.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
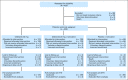
Fig 1.
CONSORT diagram. ITT, intent to treat.
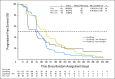
Fig 2.
Progression-free survival (PFS; based on central review postcontrast T1). No. at risk denotes the number of patients who were event free at the beginning of the period. Ced, cediranib; HR, hazard ratio; lom, lomustine; Pla, placebo.
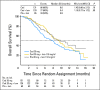
Fig 3.
Overall survival (OS). No. at risk denotes the number of patients who were event free at the beginning of the period. Ced, cediranib; HR, hazard ratio; lom, lomustine; Pla, placebo.
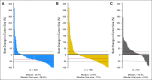
Fig 4.
Best change from baseline in contrast-enhancing area. (A) Cediranib; (B) cediranib plus lomustine; (C) placebo plus lomustine.
Comment in
- Reply to M.C. Chamberlain.
Batchelor TT, van den Bent MJ. Batchelor TT, et al. J Clin Oncol. 2014 Jul 20;32(21):2273-4. doi: 10.1200/JCO.2013.54.2878. Epub 2014 Jun 16. J Clin Oncol. 2014. PMID: 24934784 No abstract available. - Is there a role for vascular endothelial growth factor receptor 2 inhibitors in glioblastoma?
Chamberlain MC. Chamberlain MC. J Clin Oncol. 2014 Jul 20;32(21):2272. doi: 10.1200/JCO.2013.54.0088. Epub 2014 Jun 16. J Clin Oncol. 2014. PMID: 24934790 No abstract available.
Similar articles
- A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma.
Weathers SP, Han X, Liu DD, Conrad CA, Gilbert MR, Loghin ME, O'Brien BJ, Penas-Prado M, Puduvalli VK, Tremont-Lukats I, Colen RR, Yung WKA, de Groot JF. Weathers SP, et al. J Neurooncol. 2016 Sep;129(3):487-494. doi: 10.1007/s11060-016-2195-9. Epub 2016 Jul 12. J Neurooncol. 2016. PMID: 27406589 Free PMC article. Clinical Trial. - Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma.
Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, Louis DN, Cohen KS, Chea H, Exarhopoulos A, Loeffler JS, Moses MA, Ivy P, Sorensen AG, Wen PY, Jain RK. Batchelor TT, et al. J Clin Oncol. 2010 Jun 10;28(17):2817-23. doi: 10.1200/JCO.2009.26.3988. Epub 2010 May 10. J Clin Oncol. 2010. PMID: 20458050 Free PMC article. Clinical Trial. - Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial.
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ. Taal W, et al. Lancet Oncol. 2014 Aug;15(9):943-53. doi: 10.1016/S1470-2045(14)70314-6. Epub 2014 Jul 15. Lancet Oncol. 2014. PMID: 25035291 Clinical Trial. - Procarbazine, lomustine and vincristine for recurrent high-grade glioma.
Parasramka S, Talari G, Rosenfeld M, Guo J, Villano JL. Parasramka S, et al. Cochrane Database Syst Rev. 2017 Jul 26;7(7):CD011773. doi: 10.1002/14651858.CD011773.pub2. Cochrane Database Syst Rev. 2017. PMID: 28744879 Free PMC article. Review. - How did lomustine become standard of care in recurrent glioblastoma?
Weller M, Le Rhun E. Weller M, et al. Cancer Treat Rev. 2020 Jul;87:102029. doi: 10.1016/j.ctrv.2020.102029. Epub 2020 May 4. Cancer Treat Rev. 2020. PMID: 32408220 Review.
Cited by
- Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end?
Tanaka S, Louis DN, Curry WT, Batchelor TT, Dietrich J. Tanaka S, et al. Nat Rev Clin Oncol. 2013 Jan;10(1):14-26. doi: 10.1038/nrclinonc.2012.204. Epub 2012 Nov 27. Nat Rev Clin Oncol. 2013. PMID: 23183634 Review. - Antiangiogenic Therapies and Extracranial Metastasis in Glioblastoma: A Case Report and Review of the Literature.
Khattab MH, Marciscano AE, Lo SS, Lim M, Laterra JJ, Kleinberg LR, Redmond KJ. Khattab MH, et al. Case Rep Oncol Med. 2015;2015:431819. doi: 10.1155/2015/431819. Epub 2015 Jun 23. Case Rep Oncol Med. 2015. PMID: 26199775 Free PMC article. - Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages.
Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, Datta M, Amoozgar Z, Seano G, Jung K, Kamoun WS, Vardam T, Snuderl M, Goveia J, Chatterjee S, Batista A, Muzikansky A, Leow CC, Xu L, Batchelor TT, Duda DG, Fukumura D, Jain RK. Peterson TE, et al. Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4470-5. doi: 10.1073/pnas.1525349113. Epub 2016 Apr 4. Proc Natl Acad Sci U S A. 2016. PMID: 27044097 Free PMC article. - Coexisting Biopsy-Diagnosed Dementia and Glioblastoma.
Fetcko-Fayad K, Batich K, Reitman ZJ, Walsh KM, Chamberlin G, Smith V, Jones K, Cummings T, Peters KB. Fetcko-Fayad K, et al. Brain Sci. 2024 Jan 30;14(2):143. doi: 10.3390/brainsci14020143. Brain Sci. 2024. PMID: 38391718 Free PMC article. - SB365 induces apoptosis and suppresses proliferation of glioblastoma cells.
Lim JH, Jung KH, Kim MS, You JH, Park IS, Hong SS. Lim JH, et al. Indian J Pharmacol. 2020 Mar-Apr;52(2):102-107. doi: 10.4103/ijp.IJP_117_19. Epub 2020 Jun 3. Indian J Pharmacol. 2020. PMID: 32565597 Free PMC article.
References
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
- Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. - PubMed
- Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. - PubMed
- Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical