WNK1-related Familial Hyperkalemic Hypertension results from an increased expression of L-WNK1 specifically in the distal nephron - PubMed (original) (raw)
. 2013 Aug 27;110(35):14366-71.
doi: 10.1073/pnas.1304230110. Epub 2013 Aug 12.
Emilie Elvira-Matelot, Kerim Mutig, Christelle Soukaseum, Véronique Baudrie, Shengnan Wu, Lydie Cheval, Elizabeth Huc, Michèle Cambillau, Sebastian Bachmann, Alain Doucet, Xavier Jeunemaitre, Juliette Hadchouel
Affiliations
- PMID: 23940364
- PMCID: PMC3761585
- DOI: 10.1073/pnas.1304230110
WNK1-related Familial Hyperkalemic Hypertension results from an increased expression of L-WNK1 specifically in the distal nephron
Emmanuelle Vidal-Petiot et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Large deletions in the first intron of the With No lysine (K) 1 (WNK1) gene are responsible for Familial Hyperkalemic Hypertension (FHHt), a rare form of human hypertension associated with hyperkalemia and hyperchloremic metabolic acidosis. We generated a mouse model of WNK1-associated FHHt to explore the consequences of this intronic deletion. WNK1(+/FHHt) mice display all clinical and biological signs of FHHt. This phenotype results from increased expression of long WNK1 (L-WNK1), the ubiquitous kinase isoform of WNK1, in the distal convoluted tubule, which in turn, stimulates the activity of the Na-Cl cotransporter. We also show that the activity of the epithelial sodium channel is not altered in FHHt mice, suggesting that other mechanisms are responsible for the hyperkalemia and acidosis in this model. Finally, we observe a decreased expression of the renal outer medullary potassium channel in the late distal convoluted tubule of WNK1(+/FHHt) mice, which could contribute to the hyperkalemia. In summary, our study provides insights into the in vivo mechanisms underlying the pathogenesis of WNK1-mediated FHHt and further corroborates the importance of WNK1 in ion homeostasis and blood pressure.
Keywords: potassium balance; sodium transport; transgenic mouse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
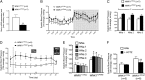
WNK1 +/FHHt mice display signs of hypervolemia. (A) Quantification of the expression level of Renin transcripts in the kidney cortex of mice WNK1 +/FHHt (n = 7) and control littermates (WNK1 +/i1lox; n = 6) by real-time RT-qPCR showed that expression of Renin is significantly decreased in WNK1 +/FHHt males (**P < 0.01). Results (mean ± SEM) are expressed in arbitrary units relative to the expression of ubc, and the expression level in WNK1 +/i1lox mice was arbitrarily set to one. (B) Profiles over 24 h in SBP under a 12:12-h day:night (7:00 AM to 7:00 PM and 7:00 PM to 7:00 AM) schedule in WNK1 +/i1lox (n = 7) and WNK1 +/FHHt (n = 6) mice kept under a control diet (NNa; 0.3% of Na+) instrumented with a telemetric system under basal condition. Data are mean ± SEM, and each point corresponds to a 1-h average. (C) SBP is increased in WNK1 +/FHHt mice (n = 6) compared with WNK1 +/i1lox littermates (n = 7). Data correspond to the 12-h night period mean ± SEM. *P < 0.05 (repeated measures two-way ANOVA). (D–F) Twelve-hour average SBP (night period) was slightly and transiently increased by administration of a high salt diet (HNa; 3% NaCl) in WNK1 +/FHHt mice, whereas the SBP of WNK1 +/i1lox mice was not affected (E). Data are mean ± SEM. *P < 0.05 compared with WNK1 +/FHHt kept under NNa (Dunnett multiple comparisons test after a one-way repeated measures ANOVA). (F) Twelve-hour average SBP (night period) was normalized in WNK1 +/FHHt mice by a hydrochlorothiazide treatment during 3 d, whereas the SBP of WNK1 +/i1lox mice was not modified. **P < 0.01 compared with WNK1 +/FHHt mice kept under a HNa (paired Student t test). For all panels, data correspond to the 12-h night period mean ± SEM.
Fig. 2.
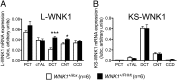
The deletion of WNK1 first intron leads to increased expression of L-WNK1 in the DCT and CNT. Quantification of the expression levels of (A) L-WNK1 and (B) KS-WNK1 transcripts in the cortical segments of the nephron of WNK1 +/i1lox (n = 6) and WNK1 +/FHHt (n = 6) mice by real-time RT-qPCR showed that expression of L-WNK1 was increased in the DCT and CNT of WNK1 +/FHHt males. Results (mean ± SEM) are expressed in arbitrary units relative to the expression of ubc. *P < 0.05; ***P < 0.001 (unpaired Student t test).
Fig. 3.
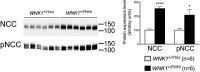
The expression, phosphorylation, and transcriptional expression of the NCC are increased in WNK1 +/FHHt mice. (Left) Representative immunoblots performed on the membrane-enriched fractions of the renal cortex of control and WNK1 +/FHHt males (n = 6 per group) incubated with anti-NCC and antiphospho-NCC (pNCC) antibodies. (Right) Densitometric analysis showed that the abundance and phosphorylation of NCC were significantly increased in WNK1 +/FHHt mice (black bars) compared with controls (open bars). Data are mean ± SEM. *P < 0.05; ****P < 0.0001 (unpaired Student t test).
Fig. 4.
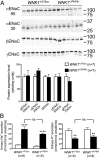
The abundance of the phosphorylated form of SPAK is not affected in homogenates of kidney cortex from WNK1 +/FHHt mice. (Left) Representative immunoblots performed on renal cortex homogenates from control and WNK1 +/FHHt males (n = 7 per group) incubated with antibodies raised against SPAK and the phosphorylated SPAK/OSR1 S motif. (Right) Densitometric analysis showed that the abundance of SPAK and its phosphorylated form on Ser343 is not modified in homogenates of cortex isolated from WNK1 +/FHHt kidneys compared with control kidneys. Data are mean ± SEM.
Fig. 5.
The expression and activity of ENaC are similar in WNK1 +/i1lox and WNK1 +/FHHt mice. (A, Upper) Representative immunoblots performed on membrane-enriched fractions of the renal cortex from control and WNK1 +/FHHt males (n = 5 per group) incubated with antibodies raised against the α-, β-, or γ-subunit of the channel. (A, Lower) Densitometric analysis showed that the abundance of the three ENaC subunits is not modified in WNK1 +/FHHt mice compared with control littermates. Data are mean ± SEM. (B) Effects of amiloride on urinary excretion of (Left) Na+ and (Right) K+ in WNK1 +/i1lox and WNK1 +/FHHt mice. One single dose of amiloride (12.5 mg/kg body weight; black bars) or vehicle (white bars) was administered i.p. to WNK1 +/i1lox (n = 8) and WNK1 +/FHHt (n = 8) mice. Urine samples were collected from 0 to 6 h after injection to measure urinary Na+ and K+ excretion. Results (mean ± SEM) are expressed as the ratio to urinary creatinine. **P < 0.01, ****P < 0.001 vs. vehicle (Bonferroni multiple comparisons test after one-way ANOVA). Ns, not significant.
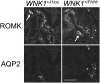
Fig. 6.
The expression of the potassium channel ROMK is decreased in the late DCT of WNK1 +/FHHt. Representative images of WNK1 +/i1lox and WNK1 +/FHHt kidneys after double labeling for ROMK and the water channel aquaporin 2 (AQP2). (Scale bar: 50 µm.) Thick ascending limb was identified by the typical strong expression of ROMK (arrows), late DCT was identified by presence of intercalated cells but weak to absent AQP2 signal, and collecting duct was identified by presence of significant AQP2 signal. Note substantially weaker ROMK signal in the late DCT and CNT of WNK1 +/FHHt kidneys compared with the controls (asterisks). Note also that the late DCT of WNK1 +/i1lox kidneys shows prominent apical ROMK signal, whereas the late DCT of WNK1 +FHHtx kidneys shows rather diffused and dotted cytoplasmic ROMK signal.
Similar articles
- Mutation affecting the conserved acidic WNK1 motif causes inherited hyperkalemic hyperchloremic acidosis.
Louis-Dit-Picard H, Kouranti I, Rafael C, Loisel-Ferreira I, Chavez-Canales M, Abdel-Khalek W, Argaiz ER, Baron S, Vacle S, Migeon T, Coleman R, Do Cruzeiro M, Hureaux M, Thurairajasingam N, Decramer S, Girerd X, O'Shaugnessy K, Mulatero P, Roussey G, Tack I, Unwin R, Vargas-Poussou R, Staub O, Grimm R, Welling PA, Gamba G, Clauser E, Hadchouel J, Jeunemaitre X. Louis-Dit-Picard H, et al. J Clin Invest. 2020 Dec 1;130(12):6379-6394. doi: 10.1172/JCI94171. J Clin Invest. 2020. PMID: 32790646 Free PMC article. - Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension.
Hadchouel J, Soukaseum C, Büsst C, Zhou XO, Baudrie V, Zürrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X. Hadchouel J, et al. Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18109-14. doi: 10.1073/pnas.1006128107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921400 Free PMC article. - Constitutively Active SPAK Causes Hyperkalemia by Activating NCC and Remodeling Distal Tubules.
Grimm PR, Coleman R, Delpire E, Welling PA. Grimm PR, et al. J Am Soc Nephrol. 2017 Sep;28(9):2597-2606. doi: 10.1681/ASN.2016090948. Epub 2017 Apr 25. J Am Soc Nephrol. 2017. PMID: 28442491 Free PMC article. - WNK1 in the kidney.
Bahena-Lopez JP, Gamba G, Castañeda-Bueno M. Bahena-Lopez JP, et al. Curr Opin Nephrol Hypertens. 2022 Sep 1;31(5):471-478. doi: 10.1097/MNH.0000000000000820. Epub 2022 Jul 15. Curr Opin Nephrol Hypertens. 2022. PMID: 35894282 Review. - A molecular update on pseudohypoaldosteronism type II.
Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P. Pathare G, et al. Am J Physiol Renal Physiol. 2013 Dec 1;305(11):F1513-20. doi: 10.1152/ajprenal.00440.2013. Epub 2013 Oct 9. Am J Physiol Renal Physiol. 2013. PMID: 24107425 Review.
Cited by
- Revisiting the NaCl cotransporter regulation by with-no-lysine kinases.
Bazúa-Valenti S, Gamba G. Bazúa-Valenti S, et al. Am J Physiol Cell Physiol. 2015 May 15;308(10):C779-91. doi: 10.1152/ajpcell.00065.2015. Epub 2015 Mar 18. Am J Physiol Cell Physiol. 2015. PMID: 25788573 Free PMC article. Review. - Hypochloraemia is strongly and independently associated with mortality in patients with chronic heart failure.
Testani JM, Hanberg JS, Arroyo JP, Brisco MA, Ter Maaten JM, Wilson FP, Bellumkonda L, Jacoby D, Tang WH, Parikh CR. Testani JM, et al. Eur J Heart Fail. 2016 Jun;18(6):660-8. doi: 10.1002/ejhf.477. Epub 2016 Jan 13. Eur J Heart Fail. 2016. PMID: 26763893 Free PMC article. - Gordon Syndrome: a continuing story.
O'Shaughnessy KM. O'Shaughnessy KM. Pediatr Nephrol. 2015 Nov;30(11):1903-8. doi: 10.1007/s00467-014-2956-7. Epub 2014 Dec 11. Pediatr Nephrol. 2015. PMID: 25503323 Review. - Role of the Cation-Chloride-Cotransporters in Cardiovascular Disease.
Meor Azlan NF, Zhang J. Meor Azlan NF, et al. Cells. 2020 Oct 14;9(10):2293. doi: 10.3390/cells9102293. Cells. 2020. PMID: 33066544 Free PMC article. Review. - Distal convoluted tubule.
McCormick JA, Ellison DH. McCormick JA, et al. Compr Physiol. 2015 Jan;5(1):45-98. doi: 10.1002/cphy.c140002. Compr Physiol. 2015. PMID: 25589264 Free PMC article. Review.
References
- Gordon RD, Hodsman GP. The syndrome of hypertension and hyperkalaemia without renal failure: Long term correction by thiazide diuretic. Scott Med J. 1986;31(1):43–44. - PubMed
- Schambelan M, Sebastian A, Rector FC., Jr Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): Role of increased renal chloride reabsorption. Kidney Int. 1981;19(5):716–727. - PubMed
- Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases