Requirement of retinoic acid receptor β for genipin derivative-induced optic nerve regeneration in adult rat retina - PubMed (original) (raw)
Requirement of retinoic acid receptor β for genipin derivative-induced optic nerve regeneration in adult rat retina
Yoshiki Koriyama et al. PLoS One. 2013.
Abstract
Like other CNS neurons, mature retinal ganglion cells (RGCs) are unable to regenerate their axons after nerve injury due to a diminished intrinsic regenerative capacity. One of the reasons why they lose the capacity for axon regeneration seems to be associated with a dramatic shift in RGCs' program of gene expression by epigenetic modulation. We recently reported that (1R)-isoPropyloxygenipin (IPRG001), a genipin derivative, has both neuroprotective and neurite outgrowth activities in murine RGC-5 retinal precursor cells. These effects were both mediated by nitric oxide (NO)/S-nitrosylation signaling. Neuritogenic activity was mediated by S-nitrosylation of histone deacetylase-2 (HDAC2), which subsequently induced retinoic acid receptor β (RARβ) expression via chromatin remodeling in vitro. RARβ plays important roles of neural growth and differentiation in development. However, the role of RARβ expression during adult rat optic nerve regeneration is not clear. In the present study, we extended this hypothesis to examine optic nerve regeneration by IPRG001 in adult rat RGCs in vivo. We found a correlation between RARβ expression and neurite outgrowth with age in the developing rat retina. Moreover, we found that IPRG001 significantly induced RARβ expression in adult rat RGCs through the S-nitrosylation of HDAC2 processing mechanism. Concomitant with RARβ expression, adult rat RGCs displayed a regenerative capacity for optic axons in vivo by IPRG001 treatment. These neuritogenic effects of IPRG001 were specifically suppressed by siRNA for RARβ. Thus, the dual neuroprotective and neuritogenic actions of genipin via S-nitrosylation might offer a powerful therapeutic tool for the treatment of RGC degenerative disorders.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
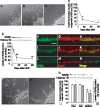
Figure 1. Correlation between axon outgrowth and RARβ expression in rat retina after birth.
(A) P1 retinal explant, (B) P14 retinal explant, (C) P60 retinal explant, Scale = 100 µm. (D) Quantification of axon outgrowth from rat retinal explants at various days after birth. Y axis shows % of explants with neurites longer than 200 µm. *P<0.01 vs P1 retina. (n = 3, 5 rats of each stage). (E) Quantification of RARβ expression in rat retina during development. *P<0.05, **P<0.01 vs P1 (n = 3). (F–N) RARβ and NeuN expression in the rat retina during development. (F, I, L) RARβ expression at P1 (F), P14 (I) and P60 (L). Scale = 50 µm. (G, J, M) NeuN-positive RGCs at P1 (G), P14 (J), and P60 (M). (H, K, N) Merged images of each day at P1 (H), P14 (K), and P60 (N). (O) P1 retina treated with scrambled siRNA (P) siRNA for RARβ in P1. Scale = 100 µm. (Q) siRNA of RARβ suppressed expression of RARβ and neurite outgrowth in P1 retina. *P<0.01 vs vehicle control (Con) and scrambled siRNA(Scr) (RARβ expression), +P<0.01 vs Con and Scr (neurite outgrowth), (n = 3, 5 rats of each treatment).
Figure 2. IPRG001 activates NADPHd staining and nNOS protein expression in the rat retina.
(A, B) NADPHd staining in the retina increased at 1 day post-treatment with 100 pmol/eye of IPRG001 (B) as compared to vehicle control (A). Scale = 100 µm. (C) Western blot analysis of nNOS protein after treatment of IPRG001. *P<0.01 vs control (n = 3). (D) Levels of nNOS expression by IPRG001 with or without optic nerve injury. *P<0.01 vs control (n = 3). (E–J) nNOS and NeuN expression in the retina after treatment of IPRG001. (E and F) Immunoreactivity of nNOS protein was increased in RGCs at 1 day after IPRG001 treatment (F) compared to vehicle control (E). Scale = 50 µm. (G and H) NeuN staining of E and F. (I and J) Merged images.
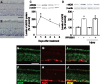
Figure 3. Upregulation of RARβ expression in RGCs after IPRG001 treatment.
(A) Western blot analysis of RARβ protein expression increased at 1 day after treatment of IPRG001. *P<0.01 vs control (n = 3). (B) Levels of RARβ expression by IPRG001 with or without optic nerve injury. *P<0.01 vs control (n = 3). (C–H) RARβ and NeuN expression in the retina after treatment of IPRG001. (C and F) Immunoreactivity of RARβ protein was increased in RGCs 1 day after treatment with IPRG001 (F) compared to vehicle control (C). Scale = 50 µm. (D and G) NeuN staining of C and F. (E and H) Merged images. (I) nNOS/NO-dependent expression of RARβ by IPRG001. *P<0.05, **P<0.01 vs control, +P<0.01 vs IPRG001 treatment alone (n = 3).
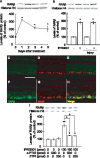
Figure 4. S-Nitrosylation of HDAC2 and the acetylation of histone H3 in the rat retina by IPRG001.
(A) S-Nitrosylation of HDAC2 1 day after intraocular administration of IPRG001. NO scavenger, c-PTIO or nNOS inhibitor, ETPI was treated 1 h before IPRG001 treatment. Biotinylated proteins were mixed with avidin beads, eluted and analyzed by western blotting with anti-HDAC2 antibody. **P<0.01 vs control. +P<0.01 vs IPRG001 treatment alone (n = 3). (B) Levels of acetylated histone H3 (AcH3) after treatment of IPRG001. *P<0.05, **P<0.01 vs control (n = 3). (C) nNOS dependent histone H3 acetylation by IPRG001. ETPI was treated 1 h before IPRG001. *P<0.01 vs control. +P<0.01 vs IPRG001 treatment alone (n = 3). (D–I) Immunohistochemistry of AcH3 and NeuN in rat RGCs. Levels of AcH3 were increased 1 day after IPRG001 treatment (G–I) compared to vehicle control (D–F). (D and G) Immunoreactivity of AcH3. (E and H) NeuN staining. (F and I) Merged images. Scale = 20 µm.
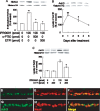
Figure 5. IPRG001-induced rat optic nerve regeneration under RARβ-dependent conditions in vivo.
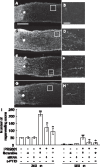
(A, C, E, G) Longitudinal sections of the adult rat optic nerve showing GAP-43 positive axons extending over the injury site (asterisks) after 2 weeks after optic nerve injury. (B, D, F, H) Enlarged image of the area enclosed within the white box of A, C, E, G. Scale = 50 µm. (A) Vehicle control, Scale = 250 µm. (C) IPRG001, (E) IPRG001 plus siRNA for RARβ. (G) IPRG001 plus c-PTIO (500 pmol). (I) Quantification of axonal regrowth at two indicated proximal points from the injury site (250 µm and 500 µm). **P<0.01 vs control, +P<0.01 vs injury plus IPRG001 (n = 8, 6 rats per each group).
Figure 6. No effect of RARβ expression on RGCs survival induced by IPRG001.
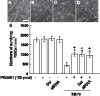
(A-D) Surviving RGCs stained by TUJ1 in whole mount retina. (A) Vehicle control. Scale = 50 µm. (B) 10 days after injury. (C) Injury plus IPRG001. (D) Injury plus IPRG001 with RARβ-specific siRNA. (E) Quantification of surviving RGCs after nerve injury with or without siRNA for RARβ. *P<0.01 vs control, +P<0.01 vs injury (n = 8).
Similar articles
- Neuritogenic activity of a genipin derivative in retinal ganglion cells is mediated by retinoic acid receptor β expression through nitric oxide/S-nitrosylation signaling.
Koriyama Y, Takagi Y, Chiba K, Yamazaki M, Arai K, Matsukawa T, Suzuki H, Sugitani K, Kagechika H, Kato S. Koriyama Y, et al. J Neurochem. 2011 Dec;119(6):1232-42. doi: 10.1111/j.1471-4159.2011.07533.x. Epub 2011 Nov 9. J Neurochem. 2011. PMID: 21995424 - Long-acting genipin derivative protects retinal ganglion cells from oxidative stress models in vitro and in vivo through the Nrf2/antioxidant response element signaling pathway.
Koriyama Y, Chiba K, Yamazaki M, Suzuki H, Muramoto K, Kato S. Koriyama Y, et al. J Neurochem. 2010 Oct;115(1):79-91. doi: 10.1111/j.1471-4159.2010.06903.x. J Neurochem. 2010. PMID: 20681953 - Neuritogenic activity of trichostatin A in adult rat retinal ganglion cells through acetylation of histone H3 lysine 9 and RARβ induction.
Koriyama Y, Sugitani K, Ogai K, Kato S. Koriyama Y, et al. J Pharmacol Sci. 2014;124(1):112-6. doi: 10.1254/jphs.13171sc. Epub 2013 Dec 27. J Pharmacol Sci. 2014. PMID: 24389816 - A molecular mechanism of optic nerve regeneration in fish: the retinoid signaling pathway.
Kato S, Matsukawa T, Koriyama Y, Sugitani K, Ogai K. Kato S, et al. Prog Retin Eye Res. 2013 Nov;37:13-30. doi: 10.1016/j.preteyeres.2013.07.004. Epub 2013 Aug 28. Prog Retin Eye Res. 2013. PMID: 23994437 Review. - Stimulating axonal regeneration of mature retinal ganglion cells and overcoming inhibitory signaling.
Fischer D. Fischer D. Cell Tissue Res. 2012 Jul;349(1):79-85. doi: 10.1007/s00441-011-1302-7. Cell Tissue Res. 2012. PMID: 22293973 Review.
Cited by
- Regulation of Myelination by Exosome Associated Retinoic Acid Release from NG2-Positive Cells.
Goncalves MB, Wu Y, Clarke E, Grist J, Hobbs C, Trigo D, Jack J, Corcoran JPT. Goncalves MB, et al. J Neurosci. 2019 Apr 17;39(16):3013-3027. doi: 10.1523/JNEUROSCI.2922-18.2019. Epub 2019 Feb 13. J Neurosci. 2019. PMID: 30760627 Free PMC article. - Genetic Advances in Microphthalmia.
Plaisancie J, Calvas P, Chassaing N. Plaisancie J, et al. J Pediatr Genet. 2016 Dec;5(4):184-188. doi: 10.1055/s-0036-1592350. Epub 2016 Sep 16. J Pediatr Genet. 2016. PMID: 27895970 Free PMC article. Review. - Toxic Advanced Glycation End-Products Inhibit Axonal Elongation Mediated by β-Tubulin Aggregation in Mice Optic Nerves.
Ooi H, Furukawa A, Takeuchi M, Koriyama Y. Ooi H, et al. Int J Mol Sci. 2024 Jul 5;25(13):7409. doi: 10.3390/ijms25137409. Int J Mol Sci. 2024. PMID: 39000515 Free PMC article. - Regenerating reptile retinas: a comparative approach to restoring retinal ganglion cell function.
Williams DL. Williams DL. Eye (Lond). 2017 Feb;31(2):167-172. doi: 10.1038/eye.2016.224. Epub 2016 Nov 11. Eye (Lond). 2017. PMID: 27834958 Free PMC article. Review. - Retinoic acid synthesis by NG2 expressing cells promotes a permissive environment for axonal outgrowth.
Goncalves MB, Wu Y, Trigo D, Clarke E, Malmqvist T, Grist J, Hobbs C, Carlstedt TP, Corcoran JPT. Goncalves MB, et al. Neurobiol Dis. 2018 Mar;111:70-79. doi: 10.1016/j.nbd.2017.12.016. Epub 2017 Dec 20. Neurobiol Dis. 2018. PMID: 29274429 Free PMC article.
References
- Bregman BS, Goldberger ME (1982) Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science 217: 553–555. - PubMed
- Goldberg JL, Klassen MP, Hua Y, Barres BA (2002) Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science 296: 1860–1864. - PubMed
- Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20: 84–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was partly supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos 25462753 to YK, 23650163 to SK and 23618006 to KS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources