Microglia/macrophage polarization dynamics in white matter after traumatic brain injury - PubMed (original) (raw)
Microglia/macrophage polarization dynamics in white matter after traumatic brain injury
Guohua Wang et al. J Cereb Blood Flow Metab. 2013 Dec.
Abstract
Mononuclear phagocytes are a population of multi-phenotypic cells and have dual roles in brain destruction/reconstruction. The phenotype-specific roles of microglia/macrophages in traumatic brain injury (TBI) are, however, poorly characterized. In the present study, TBI was induced in mice by a controlled cortical impact (CCI) and animals were killed at 1 to 14 days post injury. Real-time polymerase chain reaction (RT-PCR) and immunofluorescence staining for M1 and M2 markers were performed to characterize phenotypic changes of microglia/macrophages in both gray and white matter. We found that the number of M1-like phagocytes increased in cortex, striatum and corpus callosum (CC) during the first week and remained elevated until at least 14 days after TBI. In contrast, M2-like microglia/macrophages peaked at 5 days, but decreased rapidly thereafter. Notably, the severity of white matter injury (WMI), manifested by immunohistochemical staining for neurofilament SMI-32, was strongly correlated with the number of M1-like phagocytes. In vitro experiments using a conditioned medium transfer system confirmed that M1 microglia-conditioned media exacerbated oxygen glucose deprivation-induced oligodendrocyte death. Our results indicate that microglia/macrophages respond dynamically to TBI, experiencing a transient M2 phenotype followed by a shift to the M1 phenotype. The M1 phenotypic shift may propel WMI progression and represents a rational target for TBI treatment.
Figures
Figure 1
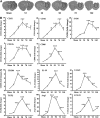
Traumatic brain injury (TBI) induced changes in messenger RNA (mRNA) expression of M1 and M2 polarization markers in injured tissue. (A) Reverse-transcription polymerase chain reaction (RT–PCR) was performed using total RNA extracted from ipsilateral striatum at 1, 3, 5, 7, and 14 days after TBI or after sham operation. The dotted black lines outline the area of striatal dissections. (B) Messenger RNA expression of M1 markers; (C) mRNA expression of M2 markers. Data are expressed as fold change versus sham-operated controls and presented as mean±standard error; _n_=6 per group; *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus sham. TGF-β, transforming growth factor β.
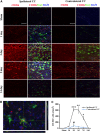
Figure 2
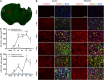
Temporal changes in microglia/macrophages expressing the M1 phenotype in cortex and striatum after traumatic brain injury (TBI). (A) Iba1 immunostaining in a coronal section of the mouse brain after TBI. The bilateral regions of interest are parietotemporal cortex (white square) and striatum (red square); (B) representative triple staining for CD16/32, Iba1, and 4',6-diamidino-2-phenylindole (DAPI) on brain sections obtained from mice at 1, 3, 5, 7, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m. Time course of bilateral CD16/32 expression in the cortex (C) and striatum (D). _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals.
Figure 3
Temporal changes in microglia/macrophages expressing the M1 phenotype in corpus callosum (CC) after traumatic brain injury (TBI). (A) Iba1 immunostaining in a coronal section of the mouse brain after TBI. The bilateral regions of interest are the CC areas (red square); (B) time course of bilateral CD16/32 expression in the CC; (C) representative triple staining for CD16/32, Iba1, and 4',6-diamidino-2-phenylindole (DAPI) in brain sections obtained from mice at 1, 3, 5, 7, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m. _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals.
Figure 4
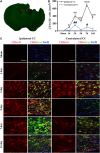
Temporal changes in microglia/macrophages expressing the M2 phenotype in cortex and striatum after traumatic brain injury (TBI). (A) Representative triple staining for CD206, Iba1, and 4',6-diamidino-2-phenylindole (DAPI) on brain sections obtained from mice at 1, 3, 5, 7, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m.Time course of bilateral CD206 expression in the cortex (B) and striatum (C). _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01 versus sham.
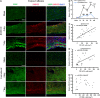
Figure 5
Temporal changes in microglia/macrophages expressing the M2 phenotype in corpus callosum (CC) after traumatic brain injury (TBI). (A) Representative triple staining for CD206, Iba1 and 4',6-diamidino-2-phenylindole (DAPI) on sections from mice at 1, 3, 5, and 14 days after TBI or sham operation. Scale bar, 50 _μ_m. (B) The higher magnification photograph shows that CD206-positive cells contain the Iba1 marker. (C) Time course of bilateral CD206 expression in the CC area. _n_=4 to 5 animals per group. *_P_⩽0.05, **P<0.01, ***P<0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals.
Figure 6
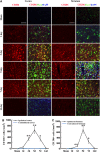
Temporal changes in white matter injury after traumatic brain injury (TBI). (A) Representative triple staining for myelin basic protein (MBP), SMI-32, and 4',6-diamidino-2-phenylindole (DAPI) in bilateral corpus callosum (CC) area in mice at 3 and 7 days after TBI or sham operation. Scale bar, 50 _μ_m. (B) Time course of bilateral SMI-32 expression in the CC area. _n_=4 to 5 animals per group. *_P_⩽0.05, **_P_⩽0.01, ***_P_⩽0.001 versus ipsilateral side of sham animals; #_P_⩽0.05 versus contralateral side of sham animals. There was a direct positive correlation between SMI-32 intensity and numbers of CD16/32+ in both ipsilateral (C) and contralateral CC (D) (all _P_⩽0.001) from mice at 1, 3, 5, and 14 days after TBI or sham operation. (E) There was no significant correlation between relative SMI-32 intensity and CD206-positive cells in ipsilateral CC (_P_=0.0547).
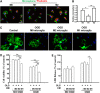
Figure 7
Effect of M1 and M2 microglia/macrophages on oxygen glucose deprivation (OGD)-induced injury in oligodendrocytes. (A)The M1 or M2 phenotypes were induced in microglial cultures using lipopolysaccharide (100 ng/mL) plus interferon-gamma (20 ng/mL) or IL-4 (20 ng/mL), respectively, for 48 hours. Microglial cells were stained with phalloidin to visualize F-actin. Scale bar, 50 _μ_m. (B) Phagocytotic activity of unstimulated (M0), M1, and M2 microglia. Phagocytosis was quantified by counting the number of phagocytosed beads in each cell. _n_=5 to 6 per group. (C) Myelin basic protein and Hoechst staining of non-OGD or OGD oligodendrocytes co-cultured with conditioned media from microglia of different phenotypes. Scale bar, 40 _μ_m. Conditioned media from microglia of different phenotypes were applied to non-OGD or post-OGD oligodendrocyte cultures for 24 hours. Neuronal survival was quantified by the MTT assay (D) and cell death was quantified by lactate dehydrogenase release (E). _n_=6 per group. All images are representative of three independent experiments. *_P_⩽0.05, **_P_⩽0.01.
Similar articles
- Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia.
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Hu X, et al. Stroke. 2012 Nov;43(11):3063-70. doi: 10.1161/STROKEAHA.112.659656. Epub 2012 Aug 28. Stroke. 2012. PMID: 22933588 - CD36 deletion prevents white matter injury by modulating microglia polarization through the Traf5-MAPK signal pathway.
Hou X, Qu X, Chen W, Sang X, Ye Y, Wang C, Guo Y, Shi H, Yang C, Zhu K, Zhang Y, Xu H, Lv L, Zhang D, Hou L. Hou X, et al. J Neuroinflammation. 2024 Jun 5;21(1):148. doi: 10.1186/s12974-024-03143-2. J Neuroinflammation. 2024. PMID: 38840180 Free PMC article. - HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis.
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, Pu H, Li WW, Tang B, Wang Y, Gao Y, Zheng P, Bennett MV, Chen J. Wang G, et al. Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2853-8. doi: 10.1073/pnas.1501441112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25691750 Free PMC article. - Microglia in the TBI brain: The good, the bad, and the dysregulated.
Loane DJ, Kumar A. Loane DJ, et al. Exp Neurol. 2016 Jan;275 Pt 3(0 3):316-327. doi: 10.1016/j.expneurol.2015.08.018. Epub 2015 Sep 3. Exp Neurol. 2016. PMID: 26342753 Free PMC article. Review. - Diversity and plasticity of microglial cells in psychiatric and neurological disorders.
Nakagawa Y, Chiba K. Nakagawa Y, et al. Pharmacol Ther. 2015 Oct;154:21-35. doi: 10.1016/j.pharmthera.2015.06.010. Epub 2015 Jun 27. Pharmacol Ther. 2015. PMID: 26129625 Review.
Cited by
- Microglia: A Potential Drug Target for Traumatic Axonal Injury.
Huang X, You W, Zhu Y, Xu K, Yang X, Wen L. Huang X, et al. Neural Plast. 2021 May 20;2021:5554824. doi: 10.1155/2021/5554824. eCollection 2021. Neural Plast. 2021. PMID: 34093701 Free PMC article. Review. - Dynamic Modulation of Microglia/Macrophage Polarization by miR-124 after Focal Cerebral Ischemia.
Hamzei Taj S, Kho W, Aswendt M, Collmann FM, Green C, Adamczak J, Tennstaedt A, Hoehn M. Hamzei Taj S, et al. J Neuroimmune Pharmacol. 2016 Dec;11(4):733-748. doi: 10.1007/s11481-016-9700-y. Epub 2016 Aug 18. J Neuroimmune Pharmacol. 2016. PMID: 27539642 Free PMC article. - Microglia subtypes show substrate- and time-dependent phagocytosis preferences and phenotype plasticity.
Li S, Wernersbach I, Harms GS, Schäfer MKE. Li S, et al. Front Immunol. 2022 Aug 29;13:945485. doi: 10.3389/fimmu.2022.945485. eCollection 2022. Front Immunol. 2022. PMID: 36105813 Free PMC article. - Targeting macrophages in cancer immunotherapy.
Duan Z, Luo Y. Duan Z, et al. Signal Transduct Target Ther. 2021 Mar 26;6(1):127. doi: 10.1038/s41392-021-00506-6. Signal Transduct Target Ther. 2021. PMID: 33767177 Free PMC article.
References
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. - PubMed
- Fork M, Bartels C, Ebert AD, Grubich C, Synowitz H, Wallesch CW. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Inj. 2005;19:101–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS43802/NS/NINDS NIH HHS/United States
- NS45048/NS/NINDS NIH HHS/United States
- NS36736,/NS/NINDS NIH HHS/United States
- R01 NS062157/NS/NINDS NIH HHS/United States
- R01 NS043802/NS/NINDS NIH HHS/United States
- R01 NS036736/NS/NINDS NIH HHS/United States
- R01 NS045048/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources