Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus - PubMed (original) (raw)
Review
Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus
Tara M Strutt et al. Immunol Rev. 2013 Sep.
Abstract
Over the last decade, the known spectrum of CD4(+) T-cell effector subsets has become much broader, and it has become clear that there are multiple dimensions by which subsets with a particular cytokine commitment can be further defined, including their stage of differentiation, their location, and, most importantly, their ability to carry out discrete functions. Here, we focus on our studies that highlight the synergy among discrete subsets, especially those defined by helper and cytotoxic function, in mediating viral protection, and on distinctions between CD4(+) T-cell effectors located in spleen, draining lymph node, and in tissue sites of infection. What emerges is a surprising multiplicity of CD4(+) T-cell functions that indicate a large arsenal of mechanisms by which CD4(+) T cells act to combat viruses.
Keywords: T-helper cells; cell differentiation; cytokines; cytotoxic T cells; infectious diseases; memory.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
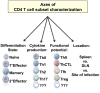
Figure 1. Defining CD4 T cell subsets
The state of activation, cytokine production profile, functional potential, and anatomical location can all be used to characterize and place CD4 T cells into distinct subsets.
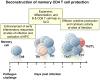
Figure 2. Phases of memory CD4 T cell protective functions
Following challenge with a pathogen, memory CD4 T cells mediate protection at different times and in different organs. Early at sites of infection, memory CD4 T cells resident in tissues, or inflammatory inducing memory T cells (ThII), enhance innate inflammatory responses and activate antigen presenting cells and other innate cells to control pathogen levels. This is followed by memory CD4 T cell provision of help for antibody producing B cells and help for CD8 T cells in the secondary lymphoid organs (SLO). At the peak of the response, memory CD4 T cells that migrate to sites of infection and produce cytokines and chemokines and kill infected cells to control and clear the pathogen.
Figure 3. Th17 effector protection does not require IL-17 production
5×106 Th17-polarized effector CD4 T cells (OT-II transgenic) recognizing IAV were transferred to either WT or IL-17 receptor-deficient (IL-17R KO) hosts. All mice were challenged with a lethal (2 LD50) dose of IAV expressing OVAII peptide and survival and weight loss monitored. Th17 effector transfer rescued both WT and IL-17R KO host survival (left), but IL-17R KO mice lost less weight (right) (n=4-6 mice/group).
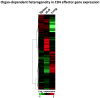
Figure 4. Secondary effectors derived from in vitro generated Th1 memory CD4 T cells show organ-specific differences in gene expression following IAV challenge
Heat map, generated using GeneSifter (GeoSpiza), showing the signal strength of genes differentially expressed by secondary CD4 T cell effectors isolated from the spleen, draining lymph node (dLN), and lung 7 days post IAV infection.
Figure 5. Compartmentalization of CD4 T cell effector functions during IAV challenge
Following activation of naive cells in the draining lymph node (dLN) by antigen presenting cells (APC) displaying viral antigen (Ag), activated effector cells develop that traffic to the spleen and lung. Functional analysis reveals that effectors present in secondary lymphoid organs (spleen and dLN) are strikingly different from the effector cells responding in the lung.
Figure 6. Compartmentalization of transcriptional regulators in CD4 T cell effectors following IAV challenge
Heat map, generated using Gene-E (Broad Institute), showing the signal strength of genes in the DAVID ontogeny pathway of transcriptional regulation differentially expressed by primary CD4 T cell effectors isolated from the spleen, draining lymph node (dLN), and lung at 7 days post infection.
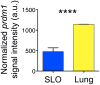
Figure 7. Differential expression of Blimp-1 in CD4 T cell effectors following IAV challenge
Relative signal of the gene encoding Blimp-1, prdm1, in CD4 T cell effectors isolated from the secondary lymphoid organs (SLO) versus the lung at 7 days post infection.
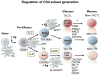
Figure 8. CD4 T cell effector subset generation
Following initial activation Bcl-6 and Blimp-1 reciprocally regulate the generation of follicular Th (Tfh and GC-Tfh) vs. non-follicular (Nfh), which include the unique ThCTL subset.
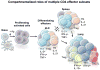
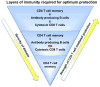
Figure 9. Layers of CD4 T cell-mediated protection
Optimal protection against IAV provided by memory CD4 T cells requires memory CD4 T cells to synergize with B cells and CD8 T cells (either through synergies with the cells themselves, or alternatively, synergies of anti-viral memory CD4 T cell functions with the distinct anti-viral functions of B cells and CD8 T cells). Protection is similarly and substantially reduced if either B cells or CD8 T cells are absent, correlating with delayed viral clearance and enhanced disease. When memory CD4 T cells respond against the same dose of virus in mice lacking both CD8 T cells and B cells, protection and viral clearance are greatly compromised.
Similar articles
- Memory CD4 T cell-mediated immunity against influenza A virus: more than a little helpful.
McKinstry KK, Dutton RW, Swain SL, Strutt TM. McKinstry KK, et al. Arch Immunol Ther Exp (Warsz). 2013 Oct;61(5):341-53. doi: 10.1007/s00005-013-0236-z. Epub 2013 May 25. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23708562 Free PMC article. Review. - Role of cytokines in the development and maintenance of memory T cells during respiratory viral infection.
Tripp RA. Tripp RA. Curr Pharm Des. 2003;9(1):51-9. doi: 10.2174/1381612033392521. Curr Pharm Des. 2003. PMID: 12570674 Review. - Short-Lived Antigen Recognition but Not Viral Infection at a Defined Checkpoint Programs Effector CD4 T Cells To Become Protective Memory.
Bautista BL, Devarajan P, McKinstry KK, Strutt TM, Vong AM, Jones MC, Kuang Y, Mott D, Swain SL. Bautista BL, et al. J Immunol. 2016 Nov 15;197(10):3936-3949. doi: 10.4049/jimmunol.1600838. Epub 2016 Oct 17. J Immunol. 2016. PMID: 27798159 Free PMC article. - Induction and function of virus-specific CD4+ T cell responses.
Whitmire JK. Whitmire JK. Virology. 2011 Mar 15;411(2):216-28. doi: 10.1016/j.virol.2010.12.015. Epub 2011 Jan 14. Virology. 2011. PMID: 21236461 Free PMC article. Review. - Immunity to Respiratory Infection Is Reinforced Through Early Proliferation of Lymphoid TRM Cells and Prompt Arrival of Effector CD8 T Cells in the Lungs.
Suarez-Ramirez JE, Chandiran K, Brocke S, Cauley LS. Suarez-Ramirez JE, et al. Front Immunol. 2019 Jun 14;10:1370. doi: 10.3389/fimmu.2019.01370. eCollection 2019. Front Immunol. 2019. PMID: 31258537 Free PMC article.
Cited by
- Comparative Investigation of Gene Regulatory Processes Underlying Avian Influenza Viruses in Chicken and Duck.
Klees S, Schlüter JS, Schellhorn J, Bertram H, Kurzweg AC, Ramzan F, Schmitt AO, Gültas M. Klees S, et al. Biology (Basel). 2022 Jan 29;11(2):219. doi: 10.3390/biology11020219. Biology (Basel). 2022. PMID: 35205087 Free PMC article. - Exposure to a mixture of 23 chemicals associated with unconventional oil and gas operations alters immune response to challenge in adult mice.
O'Dell CT, Boule LA, Robert J, Georas SN, Eliseeva S, Lawrence BP. O'Dell CT, et al. J Immunotoxicol. 2021 Dec;18(1):105-117. doi: 10.1080/1547691X.2021.1965677. J Immunotoxicol. 2021. PMID: 34455897 Free PMC article. - Timing influences the impact of aryl hydrocarbon receptor activation on the humoral immune response to respiratory viral infection.
Houser CL, Fenner KN, Lawrence BP. Houser CL, et al. Toxicol Appl Pharmacol. 2024 Aug;489:117010. doi: 10.1016/j.taap.2024.117010. Epub 2024 Jun 18. Toxicol Appl Pharmacol. 2024. PMID: 38901696 - Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines.
Sridhar S, Brokstad KA, Cox RJ. Sridhar S, et al. Vaccines (Basel). 2015 Apr 24;3(2):373-89. doi: 10.3390/vaccines3020373. Vaccines (Basel). 2015. PMID: 26343192 Free PMC article. Review. - Ex Pluribus Unum: The CD4 T Cell Response against Influenza A Virus.
Finn CM, McKinstry KK. Finn CM, et al. Cells. 2024 Apr 5;13(7):639. doi: 10.3390/cells13070639. Cells. 2024. PMID: 38607077 Free PMC article. Review.
References
- Powell TJ, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–8. - PubMed
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials