The cytotoxic necrotizing factor 1 from E. coli: a janus toxin playing with cancer regulators - PubMed (original) (raw)
Review
The cytotoxic necrotizing factor 1 from E. coli: a janus toxin playing with cancer regulators
Alessia Fabbri et al. Toxins (Basel). 2013.
Abstract
Certain strains of Escherichia coli have been indicated as a risk factor for colon cancer. E. coli is a normal inhabitant of the human intestine that becomes pathogenic, especially in extraintestinal sites, following the acquisition of virulence factors, including the protein toxin CNF1. This Rho GTPases-activating toxin induces dysfunctions in transformed epithelial cells, such as apoptosis counteraction, pro-inflammatory cytokines' release, COX2 expression, NF-kB activation and boosted cellular motility. As cancer may arise when the same regulatory pathways are affected, it is conceivable to hypothesize that CNF1-producing E. coli infections can contribute to cancer development. This review focuses on those aspects of CNF1 related to transformation, with the aim of contributing to the identification of a new possible carcinogenic agent from the microbial world.
Figures
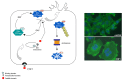
Figure 1
Mechanism of action of CNF1. CNF1 is a single chain multidomain protein toxin that contains a binding domain at the _N_-terminus, a central translocation domain, and an enzymatic domain at _C_-terminus. CNF1 exerts its deamidating activity on a glutamine residue located in the switch 2 domain of the Rho GTPases, essential for the molecule inactivation by GTP hydrolysis. CNF1, by modifying glutamine into glutamic acid, stabilizes the G proteins in their GTP-bound active form enabling them to exert a permanent activity on their effectors. By activating these GTPase, CNF1 stimulates the actin cytoskeleton, fostering a prominent ruffling activity. The activated Rho GTPases are subsequently recognized for ubiquitylation and degraded in the proteasome.
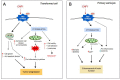
Figure 2
Signalling pathways triggered by CNF1-promoted Rho activation differ depending on the cell type. (A) In CNF1-challenged transformed cells, NF-kB translocates from cytoplasm to nucleus where it leads to the expression of pro-inflammatory and anti-apoptotic factors. Modulation of the actin cytoskeleton via the CNF1-activated Rho GTPases also plays a crucial role in certain aspects of the malignant phenotype. In particular, CNF1 induces: tumour cell motility, modification of cellular shape, loss of adhesion with consequent invasiveness and metastasis, an asymmetric cell division and aneuploidy. Furthermore, CNF1 provokes mitochondrial release of reactive oxygen species (ROS) with consequent pro-inflammatory cytokines expression. (B) In primary brain cells, through a cytoskeleton modulation, CNF1 acts on mitochondrial activity, boosts cellular ATP content, decreases pro-inflammatory cytokines expression, and increments synaptic plasticity. This leads, in vivo, to an enhancement of brain functional performances.
Similar articles
- The Rho-activating CNF1 toxin from pathogenic E. coli: a risk factor for human cancer development?
Travaglione S, Fabbri A, Fiorentini C. Travaglione S, et al. Infect Agent Cancer. 2008 Mar 12;3:4. doi: 10.1186/1750-9378-3-4. Infect Agent Cancer. 2008. PMID: 18336718 Free PMC article. - Cytotoxic necrotizing factor 1 prevents apoptosis via the Akt/IkappaB kinase pathway: role of nuclear factor-kappaB and Bcl-2.
Miraglia AG, Travaglione S, Meschini S, Falzano L, Matarrese P, Quaranta MG, Viora M, Fiorentini C, Fabbri A. Miraglia AG, et al. Mol Biol Cell. 2007 Jul;18(7):2735-44. doi: 10.1091/mbc.e06-10-0910. Epub 2007 May 16. Mol Biol Cell. 2007. PMID: 17507655 Free PMC article. - The cytotoxic necrotizing factor 1 (CNF1) from Escherichia coli.
Boquet P. Boquet P. Toxicon. 2001 Nov;39(11):1673-80. doi: 10.1016/s0041-0101(01)00154-4. Toxicon. 2001. PMID: 11595630 Review. - The Bacterial Toxin CNF1 Induces Activation and Maturation of Human Monocyte-Derived Dendritic Cells.
Gall-Mas L, Fabbri A, Namini MRJ, Givskov M, Fiorentini C, Krejsgaard T. Gall-Mas L, et al. Int J Mol Sci. 2018 May 8;19(5):1408. doi: 10.3390/ijms19051408. Int J Mol Sci. 2018. PMID: 29738516 Free PMC article. - Bacterial toxins activating Rho GTPases.
Munro P, Lemichez E. Munro P, et al. Curr Top Microbiol Immunol. 2005;291:177-90. doi: 10.1007/3-540-27511-8_10. Curr Top Microbiol Immunol. 2005. PMID: 15981464 Review.
Cited by
- CNF1-like deamidase domains: common Lego bricks among cancer-promoting immunomodulatory bacterial virulence factors.
Ho M, Mettouchi A, Wilson BA, Lemichez E. Ho M, et al. Pathog Dis. 2018 Jul 1;76(5):fty045. doi: 10.1093/femspd/fty045. Pathog Dis. 2018. PMID: 29733372 Free PMC article. Review. - Prospects of NSAIDs administration as double-edged agents against endometrial cancer and pathological species of the uterine microbiome.
Kuźmycz O, Stączek P. Kuźmycz O, et al. Cancer Biol Ther. 2020 Jun 2;21(6):486-494. doi: 10.1080/15384047.2020.1736483. Epub 2020 Mar 15. Cancer Biol Ther. 2020. PMID: 32174282 Free PMC article. Review. - The Role of Cyclomodulins and Some Microbial Metabolites in Bacterial Microecology and Macroorganism Carcinogenesis.
Markelova NN, Semenova EF, Sineva ON, Sadykova VS. Markelova NN, et al. Int J Mol Sci. 2022 Oct 3;23(19):11706. doi: 10.3390/ijms231911706. Int J Mol Sci. 2022. PMID: 36233008 Free PMC article. Review. - Gut Microbiota and Colon Cancer: A Role for Bacterial Protein Toxins?
Fiorentini C, Carlini F, Germinario EAP, Maroccia Z, Travaglione S, Fabbri A. Fiorentini C, et al. Int J Mol Sci. 2020 Aug 27;21(17):6201. doi: 10.3390/ijms21176201. Int J Mol Sci. 2020. PMID: 32867331 Free PMC article. Review. - Bacterial Toxins and Targeted Brain Therapy: New Insights from Cytotoxic Necrotizing Factor 1 (CNF1).
Tantillo E, Colistra A, Vannini E, Cerri C, Pancrazi L, Baroncelli L, Costa M, Caleo M. Tantillo E, et al. Int J Mol Sci. 2018 May 31;19(6):1632. doi: 10.3390/ijms19061632. Int J Mol Sci. 2018. PMID: 29857515 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials