Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer - PubMed (original) (raw)
Nuclear localization of the transcriptional coactivator YAP is associated with invasive lobular breast cancer
Eva J Vlug et al. Cell Oncol (Dordr). 2013 Oct.
Abstract
Background: Yes Associated Protein (YAP) has been implicated in the control of organ size by regulating cell proliferation and survival. YAP is a transcriptional coactivator that controls cellular responses through interaction with TEAD transcription factors in the nucleus, while its transcriptional functions are inhibited by phosphorylation-dependent translocation to the cytosol. YAP overexpression has been associated with different types of cancer, such as lung, skin, prostate, ovary and liver cancer. Recently, YAP was linked to E-cadherin-dependent regulation of contact inhibition in breast cancer cells.
Results: In this study we examined YAP protein expression and cellular localization in 237 cases of human invasive breast cancer by immunohistochemistry and related its expression to clinicopathological features and E-cadherin expression. We observed that invasive lobular carcinoma is characterized by higher expression levels of both nuclear and cytosolic YAP (p < 0.001). Nuclear YAP expression did not associate with other variables such as lymph node involvement, tumor grade, tumor size, mitotic activity or the molecular sub-types of invasive breast cancer. We observed that high nuclear and cytosolic YAP expression are associated with the E-cadherin deficient breast cancer subtype ILC (p < 0.001) and cell lines derived from human breast cancers and conditional mouse models of human lobular breast cancer.
Conclusions: Since our data indicate that nuclear YAP localization is more common in breast cancers lacking functional adherens junctions, it suggests that YAP-mediated transcription may be involved in the development and progression of invasive lobular breast cancer.
Figures
Fig. 1
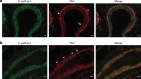
YAP expression in human and mouse normal breast tissue. a YAP expression in normal human breast tissue. Shown are immunofluorescence for YAP (middle panel, red) and E-cadherin (left panel, green). Nuclei were visualized using DAPI. Right panel depicts the merged image. Luminal epithelial cells form clear AJ and are characterized by low cytosolic YAP expression. Note the predominant nuclear YAP localization in myoepithelial cells (arrowheads) and expression of YAP in apical snouts (arrow). Size bar = 5 μm. b. YAP expression in mouse mammary glands. Shown are immunofluorescence for YAP (middle panel, red) and E-cadherin (left panel, green). Arrowheads depict nuclear YAP expression in mouse myoepithelial cells. Nuclei were visualized using DAPI. Right panel depicts the merged image. Size bar = 5 μm
Fig. 2
YAP expression in human invasive breast cancer. a Nuclear YAP expression patterns. Shown are representative examples of immunohistochemistry of YAP (IHC). The percentage of nuclei that showed YAP expression was determined and scored as 0 % (left panel) or more than 20 % (right panel). Arrows denote nuclear staining. b Cytosolic intensities of YAP expression. Shown are representative examples of immunohistochemistry of YAP (IHC). Cytosolic YAP expression was scored as either low YAP (left panel) or high YAP (right panel). The sample shown in the right panel was also scored <20 % (15–20 %) for nuclear YAP localization. Size bar = 25 μm
Fig. 3
Nuclear YAP expression is a feature of E-cadherin negative ILC. E-cadherin status correlates with nuclear YAP expression. IDC (left panels) and ILC (right panels) samples were stained for E-cadherin (top panels) and YAP (IHC) (bottom panels). Note the striking correlation between nuclear YAP and absence of E-cadherin expression. Size bar = 50 μm
Fig. 4
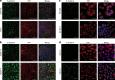
Nuclear localization of YAP in human and mouse ILC. Immunofluorescence for E-cadherin (left panels, green) and YAP (YAP-IF, middle panels, red). Nuclei were visualized using DAPI (blue). Right panels depict the merged image. In E-cadherin positive human (a) and mouse (b) cell lines YAP expression is predominantly cytosolic. E-cadherin negative human (c) and mouse ILC (d) cell lines are characterized by prominent nuclear YAP expression. Size bar = 15 μm
Similar articles
- Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer.
Vermeulen JF, van de Ven RA, Ercan C, van der Groep P, van der Wall E, Bult P, Christgen M, Lehmann U, Daniel J, van Diest PJ, Derksen PW. Vermeulen JF, et al. PLoS One. 2012;7(5):e37864. doi: 10.1371/journal.pone.0037864. Epub 2012 May 25. PLoS One. 2012. PMID: 22662240 Free PMC article. - Low shear stress induces ERK nuclear localization and YAP activation to control the proliferation of breast cancer cells.
Qin X, Li J, Sun J, Liu L, Chen D, Liu Y. Qin X, et al. Biochem Biophys Res Commun. 2019 Mar 5;510(2):219-223. doi: 10.1016/j.bbrc.2019.01.065. Epub 2019 Jan 23. Biochem Biophys Res Commun. 2019. PMID: 30685085 - YAP expression in normal and neoplastic breast tissue: an immunohistochemical study.
Jaramillo-Rodríguez Y, Cerda-Flores RM, Ruiz-Ramos R, López-Márquez FC, Calderón-Garcidueñas AL. Jaramillo-Rodríguez Y, et al. Arch Med Res. 2014 Apr;45(3):223-8. doi: 10.1016/j.arcmed.2014.01.010. Epub 2014 Mar 4. Arch Med Res. 2014. PMID: 24606817 - Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues.
Cao L, Sun PL, Yao M, Jia M, Gao H. Cao L, et al. Hum Pathol. 2017 Oct;68:166-174. doi: 10.1016/j.humpath.2017.08.032. Epub 2017 Sep 9. Hum Pathol. 2017. PMID: 28899737 Review. - Angiomotin'g YAP into the nucleus for cell proliferation and cancer development.
Hong W. Hong W. Sci Signal. 2013 Sep 3;6(291):pe27. doi: 10.1126/scisignal.2004573. Sci Signal. 2013. PMID: 24003252 Review.
Cited by
- Effect of pulsatile and continuous flow on yes-associated protein.
Chitragari G, Shalaby SY, Sumpio BJ, Sumpio BE. Chitragari G, et al. Int J Angiol. 2014 Sep;23(3):183-6. doi: 10.1055/s-0034-1376865. Int J Angiol. 2014. PMID: 25317030 Free PMC article. - Association between YAP expression in neoplastic and non-neoplastic breast tissue with arsenic urinary levels.
Michel-Ramirez G, Recio-Vega R, Ocampo-Gomez G, Palacios-Sanchez E, Delgado-Macias M, Delgado-Gaona M, Lantz RC, Gandolfi J, Gonzalez-Cortes T. Michel-Ramirez G, et al. J Appl Toxicol. 2017 Oct;37(10):1195-1202. doi: 10.1002/jat.3481. Epub 2017 May 19. J Appl Toxicol. 2017. PMID: 28524356 Free PMC article. - Sequential targeting of YAP1 and p21 enhances the elimination of senescent cells induced by the BET inhibitor JQ1.
Zhang HT, Gui T, Liu RX, Tong KL, Wu CJ, Li Z, Huang X, Xu QT, Yang J, Tang W, Sang Y, Liu W, Liu N, Ross RD, He QY, Zha ZG. Zhang HT, et al. Cell Death Dis. 2021 Jan 25;12(1):121. doi: 10.1038/s41419-021-03416-1. Cell Death Dis. 2021. PMID: 33495462 Free PMC article. - Inhibition of Yes-Associated Protein-1 (YAP1) Enhances the Response of Invasive Breast Cancer Cells to the Standard Therapy.
Guimei M, Alrouh S, Saber-Ayad M, Hafezi SA, Vinod A, Rawat S, Wardeh Y, Bakkour TM, El-Serafi AT. Guimei M, et al. Breast Cancer (Dove Med Press). 2020 Nov 2;12:189-199. doi: 10.2147/BCTT.S268926. eCollection 2020. Breast Cancer (Dove Med Press). 2020. PMID: 33173331 Free PMC article. - IMP3 Stabilization of WNT5B mRNA Facilitates TAZ Activation in Breast Cancer.
Samanta S, Guru S, Elaimy AL, Amante JJ, Ou J, Yu J, Zhu LJ, Mercurio AM. Samanta S, et al. Cell Rep. 2018 May 29;23(9):2559-2567. doi: 10.1016/j.celrep.2018.04.113. Cell Rep. 2018. PMID: 29847788 Free PMC article.
References
- Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. - DOI - PubMed
- Derksen PW, Braumuller TM, van der Burg E, Hornsveld M, Mesman E, et al. Mammary-specific inactivation of E-cadherin and p53 impairs functional gland development and leads to pleomorphic invasive lobular carcinoma in mice. Dis Model Mech. 2011;4:347–358. doi: 10.1242/dmm.006395. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous