Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values - PubMed (original) (raw)
Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values
Hyoung F Kim et al. Neuron. 2013.
Abstract
Choosing valuable objects is critical for survival, but their values may change flexibly or remain stable. Therefore, animals should be able to update the object values flexibly by recent experiences and retain them stably by long-term experiences. However, it is unclear how the brain encodes the two conflicting forms of values and controls behavior accordingly. We found that distinct circuits of the primate caudate nucleus control behavior selectively in the flexible and stable value conditions. Single caudate neurons encoded the values of visual objects in a regionally distinct manner: flexible value coding in the caudate head and stable value coding in the caudate tail. Monkeys adapted in both conditions by looking at objects with higher values. Importantly, inactivation of each caudate subregion disrupted the high-low value discrimination selectively in the flexible or stable context. This parallel complementary mechanism enables animals to choose valuable objects in both flexible and stable conditions.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
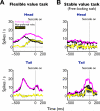
Figure 1. Learning and behavioral testing procedures for flexible and stable values
(A-B) Flexible value procedure. (A) Sequence of events in the flexible object-value association task. While the monkey was fixating on a central white dot, one of two fractal objects was presented at the neuron's preferred position. After 400 ms the fixation spot disappeared and the monkey was required to make a saccade to the object. In a block of 30-40 trials a liquid reward was delivered after the saccade to one object, but not the other object. The object-reward contingency was reversed in the next block. (B) Average target acquisition time, defined as the time from the offset of the fixation spot to the time when the eye position entered the object area (Hi, high-valued object; Lo, low-valued object). (C-D) Stable value procedure. (C) An example set of fractal objects that were associated with stable values. During long-term learning, the upper four objects were always associated with a reward (high-valued objects) while the lower four were always associated with no-reward (low-valued objects). (D) Free-looking task for testing saccades to objects with stable values. On each trial one of the eight objects in one set (as in (C)) was presented 100 or 200 ms after the fixation spot disappeared, and the monkey was free to look at it without any reward outcome (left). After long-term learning the monkey was more likely to look at the high-valued objects (right). See also Figure S1 and S2.
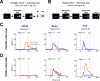
Figure 2. Flexible and stable value coding of the same three neurons in the caudate nucleus
(A) Procedure for testing neurons’ responses to objects with flexible values (same as in Figure 1A). (B) Procedure for testing neurons’ responses to objects with stable values. While the monkey was fixing on a central white dot, 2–6 fractal objects were chosen pseudorandomly from a set of 8 objects and were presented sequentially in the neuron's preferred location (each for 400 ms). The monkey was rewarded 300 ms after the final object disappeared. (C) Responses of three representative neurons recorded in the caudate head, body and tail to high-valued objects (red, 24 trials) and low-valued objects (blue, 24 trials) during the neuronal test period of flexible value procedure. They are shown by spike density functions (SDFs), which are aligned at the onset of object presentation. (D) Responses of the same three neurons shown separately for high (red, 32 trials) and low valued objects (blue, 32 trials) during the neuronal test period of stable value procedure.
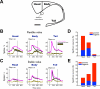
Figure 3. Differential encoding of flexible and stable values in subregions of the caudate nucleus
(A) MRI-reconstructed image of caudate nucleus and its subregions. We delineated the subregions of the caudate nucleus by the anterior commissure (head-body) and the genu (body-tail). Scale bar indicates 5 mm. (B) Average responses to the flexibly valued objects of neurons in the head (n = 159), body (n = 88) and tail (n = 102) of the caudate nucleus. Neuronal responses were averaged for the neurons’ preferred values (magenta) and non-preferred values (black) using a cross-validation method (see supplementary information). The yellow line indicates the difference between the preferred and non-preferred responses (mean ± SE). (C) Average responses to the stably valued objects in the three caudate subregions (head, n = 129; body, n = 86; tail, n = 92). (D) Proportions of flexible value coding neurons in the three caudate subregions. Red: positive neurons that responded more strongly to high-valued objects. Blue: negative neurons that responded more strongly to low-valued objects. (E) Proportions of stable value coding neurons. See also Figure S3 and S4.
Figure 4. Flexible and stable values by individual neurons in the caudate subregions
(A-C) Comparison between flexible value coding (abscissa) and stable value coding (ordinate) in the caudate head (n=125), body (n=65) and tail (n=87). Plotted for each neuron (each dot) are the magnitudes of the flexible and stable value coding measured by ROC areas. ROC areas higher and lower than 0.5 indicate positive and negative value coding, respectively. Purple: neurons encoding only flexible values. Black: neurons encoding only stable values. Green: neurons encoding both flexible and stable values. White: neurons encoding neither value.
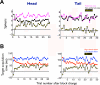
Figure 5. Time courses of the neuronal and behavioral responses in the flexible value procedure, shown separately for the caudate head and tail
(A) Neuronal responses plotted against the number of trials after the reversal of the object-reward contingency. The responses were averaged for the neurons’ preferred values (magenta) and non-preferred values (black). Yellow dotted line indicates the difference between the preferred and non-preferred responses. (B) Behavioral responses while the neuronal responses were obtained in the caudate head and tail. The target acquisition times were averaged for the high valued object (red) and low valued object (blue). Yellow dotted line indicates the difference of target acquisition time between the high and low valued objects.
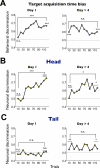
Figure 6. Changes in the neuronal and behavioral responses during the combination of short-term and long-term learning of object values
The monkey performed the object value learning task (Figure S2) in two stages of long-term learning: 1) initial stage where novel objects were used as the saccade target (Day 1), and 2) late stage where objects had been learned more than 4 times (Day > 4). (A) Behavioral discrimination based on the target acquisition time. (B) Caudate head neuronal discrimination (Day 1, n = 18; Day > 4, n = 23). (C) Caudate tail neuronal discrimination (Day 1, n = 29; Day > 4, n = 21). Each graph shows the short-term changes in the discrimination within a session of the object value learning (number of trials: 120). Each data point indicates the discrimination in a sub-block of 10 trials, computed as an ROC area. For the behavioral data (A) an ROC area larger than 0.5 indicates a stronger preference of high-valued objects. For the neuronal data (B and C) the ROC areas were normalized so that the neuron's preferred value is plotted upward. Specifically, if the original ROC areas showed a negative slope during learning, the values were flipped with respect to 0.5 (i.e., neutral value). The statistical significance of the discrimination was tested for the early and late stages of the short-term learning (1 sub-block = 10 trials for behavioral discrimination, 2 sub-blocks = 20 trials for neuronal discrimination) in two ways: 1) comparison with the neutral value (ROC 0.5), and 2) comparison between the early and late stages. * p<0.05, ** p<0.01, *** p<0.001, n.s. non-significant.
Figure 7. Presaccadic neuronal activity in the flexible and stable value procedure, shown separately for the caudate head and tail
(A) Averaged neuronal activity aligned on the onsets of saccades to preferred values (magenta) and non-preferred values (black) in the flexible value procedure (Figure 1A). The yellow line indicates the difference in neuronal activity between the preferred and non-preferred values (caudate head, n = 159; tail, n = 102; mean ± SE). (B) Averaged neuronal activity aligned on the onsets of saccades to preferred values (magenta) and non-preferred values (black) in the stable value procedure (free-looking task, Figure 1D). The yellow line indicates the difference in neuronal activity (caudate head, n = 20; tail, n = 20; mean ± SE). See also figure S5.
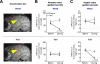
Figure 8. Differential impairments of flexible and stable value-guided saccades by caudate head and tail inactivations
(A) Example injection sites of muscimol in the caudate nucleus (yellow structure) reconstructed on an MR image: caudate head (top) and tail (bottom). Scale bar indicates 5 mm. (B) Effects on the controlled saccades in the flexible value procedure (Figure 1B). The differences in the target acquisition time between high- and low-valued objects are plotted before and during inactivation (mean ± SE). Data are shown separately for caudate head inactivation (top, n = 6) and tail inactivation (bottom, n = 9), and for contralateral saccades (solid line) and ipsilateral saccades (dashed line). (C) Effects on the automatic saccades in the stable value procedure (free-looking task, Figure 1D). The differences in the probability of automatic looking between high- and low-valued objects are plotted before and during inactivation (mean ± SE). Same format as in (B). See also figure S6-8.
Similar articles
- Basal ganglia circuits for reward value-guided behavior.
Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Hikosaka O, et al. Annu Rev Neurosci. 2014;37:289-306. doi: 10.1146/annurev-neuro-071013-013924. Annu Rev Neurosci. 2014. PMID: 25032497 Free PMC article. Review. - What and where information in the caudate tail guides saccades to visual objects.
Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. Yamamoto S, et al. J Neurosci. 2012 Aug 8;32(32):11005-16. doi: 10.1523/JNEUROSCI.0828-12.2012. J Neurosci. 2012. PMID: 22875934 Free PMC article. - Direct and indirect pathways for choosing objects and actions.
Hikosaka O, Kim HF, Amita H, Yasuda M, Isoda M, Tachibana Y, Yoshida A. Hikosaka O, et al. Eur J Neurosci. 2019 Mar;49(5):637-645. doi: 10.1111/ejn.13876. Epub 2018 Mar 25. Eur J Neurosci. 2019. PMID: 29473660 Free PMC article. Review. - Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement.
Shimo Y, Hikosaka O. Shimo Y, et al. J Neurosci. 2001 Oct 1;21(19):7804-14. doi: 10.1523/JNEUROSCI.21-19-07804.2001. J Neurosci. 2001. PMID: 11567071 Free PMC article. - Representation of outcome risk and action in the anterior caudate nucleus.
Yanike M, Ferrera VP. Yanike M, et al. J Neurosci. 2014 Feb 26;34(9):3279-90. doi: 10.1523/JNEUROSCI.3818-13.2014. J Neurosci. 2014. PMID: 24573287 Free PMC article.
Cited by
- Neurons in the primate dorsal striatum signal the uncertainty of object-reward associations.
White JK, Monosov IE. White JK, et al. Nat Commun. 2016 Sep 14;7:12735. doi: 10.1038/ncomms12735. Nat Commun. 2016. PMID: 27623750 Free PMC article. - Neural signatures of experience-based improvements in deterministic decision-making.
Tremel JJ, Laurent PA, Wolk DA, Wheeler ME, Fiez JA. Tremel JJ, et al. Behav Brain Res. 2016 Dec 15;315:51-65. doi: 10.1016/j.bbr.2016.08.023. Epub 2016 Aug 11. Behav Brain Res. 2016. PMID: 27523644 Free PMC article. - Rethinking Vision and Action.
Nakayama K, Moher J, Song JH. Nakayama K, et al. Annu Rev Psychol. 2023 Jan 18;74:59-86. doi: 10.1146/annurev-psych-021422-043229. Annu Rev Psychol. 2023. PMID: 36652303 Free PMC article. Review. - A key role for stimulus-specific updating of the sensory cortices in the learning of stimulus-reward associations.
van den Berg B, Geib BR, San Martín R, Woldorff MG. van den Berg B, et al. Soc Cogn Affect Neurosci. 2019 Feb 13;14(2):173-187. doi: 10.1093/scan/nsy116. Soc Cogn Affect Neurosci. 2019. PMID: 30576533 Free PMC article. - Differential functional dysconnectivity of caudate nucleus subdivisions in Parkinson's disease.
Jia X, Pan Z, Chen H, Wang Z, Li K, Wang X, Wang Z, Ma H, Feng T, Yang Q. Jia X, et al. Aging (Albany NY). 2020 Sep 5;12(16):16183-16194. doi: 10.18632/aging.103628. Aging (Albany NY). 2020. PMID: 32687066 Free PMC article.
References
- Abraham WC, Robins A. Memory retention – the synaptic stability versus plasticity dilemma. Trends Neurosci. 2005;28:73–78. - PubMed
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. - PubMed
- Anderson P. Flexibility and stability in the innovating economy. Admin. Sci. Quart. 2007;52:333–335.
- Ashby FG, Maddox WT. Human category learning. Annu. Rev. Neurosci. 2005;56:149–178. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources