The case for immunomodulatory approaches in treating HSV encephalitis - PubMed (original) (raw)
The case for immunomodulatory approaches in treating HSV encephalitis
Chandran Ramakrishna et al. Future Virol. 2013.
Abstract
HSV encephalitis (HSE) is the most prevalent sporadic viral encephalitis. Although safe and effective antiviral therapies and greatly improved noninvasive diagnostic procedures have significantly improved outcomes, mortality (~20%) and debilitating neurological sequelae in survivors remain unacceptably high. An encouraging new development is that the focus is now shifting away from the virus exclusively, to include consideration of the host immune response to infection in the pathology underlying development of HSE. In this article, the authors discuss results from recent studies in experimental mouse models, as well as clinical reports that demonstrate a role for exaggerated host inflammatory responses in the brain in the development of HSE that is motivating researchers and clinicians to consider new therapeutic approaches for treating HSE. The authors also discuss results from a few studies that have shown that immunomodulatory drugs can be highly protective against HSE, which supports a role for deleterious host inflammatory responses in HSE. The impressive outcomes of some immunomodulatory approaches in mouse models of HSE emphasize the urgent need for clinical trials to rigorously evaluate combination antiviral and immunomodulatory therapy in comparison with standard antiviral therapy for treatment of HSE, and support for such an initiative is gaining momentum.
Keywords: acyclovir; encephalitis; herpesvirus; immune pathology; immunomodulation; inflammation; innate immunity; intravenous immunoglobulin; reactive oxygen species.
Conflict of interest statement
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
Figure 1. EEG (left) and brain MRI (right) fluid-attenuated inversion recovery in a 46-year-old man who developed abrupt confusion 8 months after allogeneic hematopoietic cell transplantation for acute myelogenous leukemia
EEG showed right-sided periodic sharp waves and MRI showed increased signal in the right hippocampus. Cerebrospinal fluid had 44 white blood cells/µl (87% lymphocytes) and 11 red blood cells/µl. HSV-1 was cultured from the cerebrospinal fluid. Despite treatment with acyclovir and then foscarnet, the patient became amnesic, unable to form new memory traces. L: Left; R: Right. Reproduced with permission from [7].
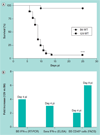
Figure 2. Hematopoietic cells control decisions on survival
Irradiated 129 Rag-knockout mice received B6 BM while nonirradiated 129 Rag-knockout recipients were transferred with splenocytes isolated from either C57BL/6 (B6) or 129 WT mice. Mice were infected with 3200 PFU HSV 17+ and observed for mortality. n = 6–10; ***p < 0.0005 for B6 versus 129. BM: Bone marrow; pi: Postinfection; WT: Wild-type.
Figure 3. Increased inflammation in susceptible 129 mice
(A) 129 mice are highly susceptible to HSV 17+ infection compared with B6 mice. (B) Type I IFN transcripts were measured by RT-PCR in BS (BS IFN-α) or protein in sera by ELISA (sera IFN-α) at day 4 pi. CD45hi inflammatory cells in the BS were detected by flow cytometry (BS CD45hi cells) at days 4 and 8 pi. ****p < 0.0001. BS: Brainstem; FACS: Fluorescence-activated cell sorting; pi: Postinfection; RT-PCR: Real-time PCR; WT: Wild-type.
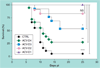
Figure 4. Acyclovir administered early but not late is protective
A 7-day ACV course of treatment was started 48 h (D2), 72–80 h (D3) or 96 h (D4) pi after inoculation of mice with HSV 17+; mice were monitored daily for mortality and symptoms of HSV-1 encephalitis necessitating euthanasia. CTRLs: phosphate-buffered saline; n = 12–20 129S6 mice. ACV-D4 versus CTRL = NS. *p = 0.032; **p = 0.002; ****p < 0.00001. ACV: Acyclovir; CTRL: Control; D: Day; NS: Nonsignificant; pi: Postinfection.
Figure 5. HSV pathogenesis in mice
Susceptible mice: increased inflammation in BS (lesion hyperintensity in MRI – yellow arrow) and virus spread to cerebrum and spinal cord. Resistant mice: reduced inflammation in BS (no lesion hyperintensity in MRI – yellow box) and limited spread to cerebrum and spinal cord. BS: Brainstem. MRI image reproduced with permission from [31] © American Society for Microbiology (2008).
Similar articles
- Immunomodulatory Strategies in Herpes Simplex Virus Encephalitis.
Piret J, Boivin G. Piret J, et al. Clin Microbiol Rev. 2020 Feb 12;33(2):e00105-19. doi: 10.1128/CMR.00105-19. Print 2020 Mar 18. Clin Microbiol Rev. 2020. PMID: 32051176 Free PMC article. Review. - Innate immune response during herpes simplex virus encephalitis and development of immunomodulatory strategies.
Piret J, Boivin G. Piret J, et al. Rev Med Virol. 2015 Sep;25(5):300-19. doi: 10.1002/rmv.1848. Epub 2015 Jul 23. Rev Med Virol. 2015. PMID: 26205506 Review. - Herpes Simplex Encephalitis: an Update.
Gnann JW Jr, Whitley RJ. Gnann JW Jr, et al. Curr Infect Dis Rep. 2017 Mar;19(3):13. doi: 10.1007/s11908-017-0568-7. Curr Infect Dis Rep. 2017. PMID: 28251511 Review. - Herpes simplex encephalitis.
Sköldenberg B. Sköldenberg B. Scand J Infect Dis Suppl. 1996;100:8-13. Scand J Infect Dis Suppl. 1996. PMID: 9163027 Review.
Cited by
- Establishment of HSV1 latency in immunodeficient mice facilitates efficient in vivo reactivation.
Ramakrishna C, Ferraioli A, Calle A, Nguyen TK, Openshaw H, Lundberg PS, Lomonte P, Cantin EM. Ramakrishna C, et al. PLoS Pathog. 2015 Mar 11;11(3):e1004730. doi: 10.1371/journal.ppat.1004730. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25760441 Free PMC article. - Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis.
Ramakrishna C, Kujawski M, Chu H, Li L, Mazmanian SK, Cantin EM. Ramakrishna C, et al. Nat Commun. 2019 May 14;10(1):2153. doi: 10.1038/s41467-019-09884-6. Nat Commun. 2019. PMID: 31089128 Free PMC article. - Effects of Acyclovir and IVIG on Behavioral Outcomes after HSV1 CNS Infection.
Ramakrishna C, Golub MS, Chiang A, Hong T, Kalkum M, Cantin EM. Ramakrishna C, et al. Behav Neurol. 2017;2017:5238402. doi: 10.1155/2017/5238402. Epub 2017 Nov 19. Behav Neurol. 2017. PMID: 29358844 Free PMC article. - Intravenous immunoglobulin for the treatment of childhood encephalitis.
Iro MA, Martin NG, Absoud M, Pollard AJ. Iro MA, et al. Cochrane Database Syst Rev. 2017 Oct 2;10(10):CD011367. doi: 10.1002/14651858.CD011367.pub2. Cochrane Database Syst Rev. 2017. PMID: 28967695 Free PMC article. Review. - Serotypes of human enteroviruses causing pediatric viral encephalitis and meningitis in Hebei province, China, from 2013 to 2015.
Chen X, Guo J, Li J, Li Q, Ai J, Sun S, Xie Z. Chen X, et al. Pediatr Investig. 2018 Jul 16;2(2):98-104. doi: 10.1002/ped4.12037. eCollection 2018 Jun. Pediatr Investig. 2018. PMID: 32851241 Free PMC article.
References
- Cabrera CV, Wohlenberg C, Openshaw H, Rey-Mendez M, Puga A, Notkins AL. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature. 1980;288(5788):288–290. - PubMed
- Yao H-W, Ling P, Chen S-H, Tung Y-Y, Chen S-H. Factors affecting herpes simplex virus reactivation from the explanted mouse brain. Virology. 2012;433(1):116–123. ▪ This study provides convincing evidence for HSV-1 reactivation in brainstem.
- Whitley R. Herpes virus infections of the central nervous system. Herpes. 2003;11(Suppl. 2):1–84.
Website
LinkOut - more resources
Full Text Sources
Other Literature Sources