Viral subversion of the nuclear pore complex - PubMed (original) (raw)
Review
Viral subversion of the nuclear pore complex
Valerie Le Sage et al. Viruses. 2013.
Abstract
The nuclear pore complex (NPC) acts as a selective barrier between the nucleus and the cytoplasm and is responsible for mediating communication by regulating the transport of RNA and proteins. Numerous viral pathogens have evolved different mechanisms to hijack the NPC in order to regulate trafficking of viral proteins, genomes and even capsids into and out of the nucleus thus promoting virus replication. The present review examines the different strategies and the specific nucleoporins utilized during viral infections as a means of promoting their life cycle and inhibiting host viral defenses.
Figures
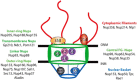
Figure 1
The nuclear pore complex (NPC). Schematic representation of a cross-section view of a NPC as it spans the inner (INM) and outer (ONM) nuclear membranes. Vertebrate Nups are listed based on their localization within the NPC. The central core (green) is composed of the inner-ring Nups, transmembrane Nups, linker Nups, outer-ring Nups and the phenylalanine-glycine (FG)-Nups. The location of select nucleoporins within the NPC is shown.
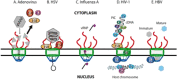
Figure 2
Viruses that hijack the nuclear pore complex to promote viral genome nuclear import. Schematic representations of the different mechanisms by which 5 viruses subvert various Nups in order to facilitate nuclear import. (A) Uncoating of the adenovirus capsid at the NPC causes at once the release of the DNA genome into the nucleus and displacement of Nup62, Nup214 and Nup358 into the cytoplasm. (B) HSV-1 capsid docking to cytoplasmic filaments leads to uncoating and driving the DNA genome through the NPC. (C) Import of the influenza A virus vRNP is mediated by the classical importin α/β transport pathway. (D) The HIV-1 PIC (pre-integration complex) docks to Nup358 before being imported into the nucleus and integrated into the host chromosome in the process that is facilitated by Nup62, Nup98 and Nup153. (E) Mature and immature HBV capsids are imported intact through the NPC, however; Nup153 ensures that only mature capsids disintegrate to release the DNA genome into the nucleus.
Figure 3
Changes to the NPC by viruses during nuclear export. Schematic representations of the different mechanisms by which 6 viruses subvert various Nups in order to facilitate nuclear export. (A) HSV-1 capsids exit the nucleus via a process of primary envelopment and de-envelopment. Secondary effects include dilation of the NPC channel and redistribution of Nup153. (B) Enteroviruses replicate in the cytoplasm and induce degradation of Nup62, Nup98 and Nup153 by the protease 2Apro (scissors). (C) Cardiovirus infection induces hyperphosphorylation (P) of Nup62, Nup98, Nup153 and Nup214. (D) Influenza A downregulates the expression of Nup98 and relocalizes Nup62 to the cytoplasm. (E) VSV M protein inhibits mRNA export through an interaction with the Rae1-Nup98 complex in the nucleoplasm. (F) HIV-1 Rev-mediated vRNA export is CRM1-dependent and causes the displacement of Nup62 into the cytoplasm.
Similar articles
- Viruses challenge selectivity barrier of nuclear pores.
Labokha AA, Fassati A. Labokha AA, et al. Viruses. 2013 Sep 30;5(10):2410-23. doi: 10.3390/v5102410. Viruses. 2013. PMID: 24084236 Free PMC article. Review. - Nuclear import in viral infections.
Greber UF, Fornerod M. Greber UF, et al. Curr Top Microbiol Immunol. 2005;285:109-38. doi: 10.1007/3-540-26764-6_4. Curr Top Microbiol Immunol. 2005. PMID: 15609502 Review. - BGLF4 kinase modulates the structure and transport preference of the nuclear pore complex to facilitate nuclear import of Epstein-Barr virus lytic proteins.
Chang CW, Lee CP, Su MT, Tsai CH, Chen MR. Chang CW, et al. J Virol. 2015 Feb;89(3):1703-18. doi: 10.1128/JVI.02880-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410863 Free PMC article. - The Viral Capsid: A Master Key to Access the Host Nucleus.
Blanco-Rodriguez G, Di Nunzio F. Blanco-Rodriguez G, et al. Viruses. 2021 Jun 20;13(6):1178. doi: 10.3390/v13061178. Viruses. 2021. PMID: 34203080 Free PMC article. Review. - Nucleocytoplasmic Trafficking Perturbation Induced by Picornaviruses.
Lizcano-Perret B, Michiels T. Lizcano-Perret B, et al. Viruses. 2021 Jun 23;13(7):1210. doi: 10.3390/v13071210. Viruses. 2021. PMID: 34201715 Free PMC article. Review.
Cited by
- Viruses challenge selectivity barrier of nuclear pores.
Labokha AA, Fassati A. Labokha AA, et al. Viruses. 2013 Sep 30;5(10):2410-23. doi: 10.3390/v5102410. Viruses. 2013. PMID: 24084236 Free PMC article. Review. - Interaction of adenovirus type 5 E4orf4 with the nuclear pore subunit Nup205 is required for proper viral gene expression.
Lu Y, Kucharski TJ, Gamache I, Blanchette P, Branton PE, Teodoro JG. Lu Y, et al. J Virol. 2014 Nov;88(22):13249-59. doi: 10.1128/JVI.00933-14. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210169 Free PMC article. - Nuclear Periphery Takes Center Stage: The Role of Nuclear Pore Complexes in Cell Identity and Aging.
Cho UH, Hetzer MW. Cho UH, et al. Neuron. 2020 Jun 17;106(6):899-911. doi: 10.1016/j.neuron.2020.05.031. Neuron. 2020. PMID: 32553207 Free PMC article. Review. - Architecture of the symmetric core of the nuclear pore.
Lin DH, Stuwe T, Schilbach S, Rundlet EJ, Perriches T, Mobbs G, Fan Y, Thierbach K, Huber FM, Collins LN, Davenport AM, Jeon YE, Hoelz A. Lin DH, et al. Science. 2016 Apr 15;352(6283):aaf1015. doi: 10.1126/science.aaf1015. Epub 2016 Apr 14. Science. 2016. PMID: 27081075 Free PMC article. - Antiviral Immune Response as a Trigger of FUS Proteinopathy in Amyotrophic Lateral Sclerosis.
Shelkovnikova TA, An H, Skelt L, Tregoning JS, Humphreys IR, Buchman VL. Shelkovnikova TA, et al. Cell Rep. 2019 Dec 24;29(13):4496-4508.e4. doi: 10.1016/j.celrep.2019.11.094. Cell Rep. 2019. PMID: 31875556 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources