Premotor cortex is critical for goal-directed actions - PubMed (original) (raw)
Premotor cortex is critical for goal-directed actions
Christina M Gremel et al. Front Comput Neurosci. 2013.
Abstract
Shifting between motor plans is often necessary for adaptive behavior. When faced with changing consequences of one's actions, it is often imperative to switch from automatic actions to deliberative and controlled actions. The pre-supplementary motor area (pre-SMA) in primates, akin to the premotor cortex (M2) in mice, has been implicated in motor learning and planning, and action switching. We hypothesized that M2 would be differentially involved in goal-directed actions, which are controlled by their consequences vs. habits, which are more dependent on their past reinforcement history and less on their consequences. To investigate this, we performed M2 lesions in mice and then concurrently trained them to press the same lever for the same food reward using two different schedules of reinforcement that differentially bias towards the use of goal-directed versus habitual action strategies. We then probed whether actions were dependent on their expected consequence through outcome revaluation testing. We uncovered that M2 lesions did not affect the acquisition of lever-pressing. However, in mice with M2 lesions, lever-pressing was insensitive to changes in expected outcome value following goal-directed training. However, habitual actions were intact. We confirmed a role for M2 in goal-directed but not habitual actions in separate groups of mice trained on the individual schedules biasing towards goal-directed versus habitual actions. These data indicate that M2 is critical for actions to be updated based on their consequences, and suggest that habitual action strategies may not require processing by M2 and the updating of motor plans.
Keywords: action selection; goal-directed actions; habitual actions; premotor cortex; value-based decision making.
Figures
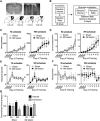
Figure 1
M2 lesions disrupt the ability to shift between automatic and goal-directed actions in the same animal. Representative picture of an M2 lesion (A), with the area of lesion outlined in the box in the left panel enlarged in the right panel. Bottom panels are illustrated examples showing approximately the largest (black) and smallest (grey) extent of the lesions observed. (B) Schematic of experimental design. During acquisition, mice were concurrently trained under random interval (RI) and random ratio (RR) reinforcement schedules to press a similar lever for the same outcome. A separate outcome was provided daily in the home cage. Mice then underwent outcome revaluation testing comprising Valued (pre-fed home-cage outcome) and Devalued (pre-fed operant outcome) days. (C–F) Lever-pressing behavior during acquisition of concurrent RI (left panel) and RR (right panel) schedules, showing the effects of M2 lesions on the number of lever presses made (C), the rate of lever pressing (D), the number of rewards earned (E), or head entries (F) during RI and RR schedule training. Mice then underwent subsequent outcome revaluation testing. (G) Shown is normalized lever pressing [Lever presses for each Revaluation state (Valued or Devalued state)/total Lever presses (Valued + Devalued states)] during outcome revaluation testing for Sham and M2 lesion mice. Non-reinforced lever pressing in previously RI and RR training contexts was examined on both Valued (black bars) and Devalued (grey bars) days. Error bars = ± SEM. * = Bonferroni corrected p < 0.05.
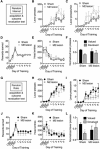
Figure 2
M2 lesions disrupt goal-directed actions but spare habitual actions. Separate groups of mice were trained to lever press for an outcome under only RI or only RR schedules of reinforcement, and then underwent subsequent outcome revaluation testing. (A) Schematic of experimental design. Mice were trained to press a lever only under a random interval (RI) reinforcement schedule, and then underwent outcome revaluation testing. (B–F) Effect of M2 lesions on acquisition under RI schedule on the average number of lever-presses made (B), the average rate of lever-pressing (C), the average rewards earned (D), and the average head entries performed (E). (F) Effect of outcome revaluation on normalized lever-pressing following RI schedule training for Sham and M2 lesion mice. (G) Schematic of experimental design. Mice were trained to press a lever only under a random ratio (RR) reinforcement schedule, and then underwent outcome revaluation testing (H–K) Effect of M2 lesions on acquisition under RR schedule on the average number of lever-presses made (H), the average rate of lever-pressing (I), the average rewards earned (J), and the average head entries performed (K). (L) Effect of outcome revaluation on normalized lever-pressing following RR schedule training for Sham and M2 lesion mice. For revaluation testing, Valued days = black bars, and Devalued day = grey bars. Error bars = ± SEM. * = Bonferroni corrected p < 0.05.
Similar articles
- Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions.
Gremel CM, Costa RM. Gremel CM, et al. Nat Commun. 2013;4:2264. doi: 10.1038/ncomms3264. Nat Commun. 2013. PMID: 23921250 Free PMC article. - Targeting the lateral hypothalamus with short hairpin RNAs reduces habitual behaviour following extended instrumental training in rats.
Bingul A, Merlin S, Carrive P, Killcross S, Furlong TM. Bingul A, et al. Neurobiol Learn Mem. 2022 Sep;193:107657. doi: 10.1016/j.nlm.2022.107657. Epub 2022 Jul 2. Neurobiol Learn Mem. 2022. PMID: 35792325 - Habitual Behavior Is Mediated by a Shift in Response-Outcome Encoding by Infralimbic Cortex.
Barker JM, Glen WB, Linsenbardt DN, Lapish CC, Chandler LJ. Barker JM, et al. eNeuro. 2018 Jan 3;4(6):ENEURO.0337-17.2017. doi: 10.1523/ENEURO.0337-17.2017. eCollection 2017 Nov-Dec. eNeuro. 2018. PMID: 29302616 Free PMC article. - Habits, action sequences and reinforcement learning.
Dezfouli A, Balleine BW. Dezfouli A, et al. Eur J Neurosci. 2012 Apr;35(7):1036-51. doi: 10.1111/j.1460-9568.2012.08050.x. Eur J Neurosci. 2012. PMID: 22487034 Free PMC article. Review. - Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action.
Schwabe L, Wolf OT. Schwabe L, et al. Behav Brain Res. 2011 Jun 1;219(2):321-8. doi: 10.1016/j.bbr.2010.12.038. Epub 2011 Jan 8. Behav Brain Res. 2011. PMID: 21219935 Review.
Cited by
- Strengthened Inputs from Secondary Motor Cortex to Striatum in a Mouse Model of Compulsive Behavior.
Corbit VL, Manning EE, Gittis AH, Ahmari SE. Corbit VL, et al. J Neurosci. 2019 Apr 10;39(15):2965-2975. doi: 10.1523/JNEUROSCI.1728-18.2018. Epub 2019 Feb 8. J Neurosci. 2019. PMID: 30737313 Free PMC article. - Reconstruction of Intratelencephalic Neurons in the Mouse Secondary Motor Cortex Reveals the Diverse Projection Patterns of Single Neurons.
Lin HM, Kuang JX, Sun P, Li N, Lv X, Zhang YH. Lin HM, et al. Front Neuroanat. 2018 Oct 30;12:86. doi: 10.3389/fnana.2018.00086. eCollection 2018. Front Neuroanat. 2018. PMID: 30425624 Free PMC article. - Chronic alcohol exposure alters action control via hyperactive premotor corticostriatal activity.
Schreiner DC, Wright A, Baltz ET, Wang T, Cazares C, Gremel CM. Schreiner DC, et al. Cell Rep. 2023 Jul 25;42(7):112675. doi: 10.1016/j.celrep.2023.112675. Epub 2023 Jun 20. Cell Rep. 2023. PMID: 37342908 Free PMC article. - Concomitant Processing of Choice and Outcome in Frontal Corticostriatal Ensembles Correlates with Performance of Rats.
Handa T, Harukuni R, Fukai T. Handa T, et al. Cereb Cortex. 2021 Jul 29;31(9):4357-4375. doi: 10.1093/cercor/bhab091. Cereb Cortex. 2021. PMID: 33914862 Free PMC article. - Pars Opercularis Underlies Efferent Predictions and Successful Auditory Feedback Processing in Speech: Evidence From Left-Hemisphere Stroke.
Beach SD, Tang DL, Kiran S, Niziolek CA. Beach SD, et al. Neurobiol Lang (Camb). 2024 Jun 3;5(2):454-483. doi: 10.1162/nol_a_00139. eCollection 2024. Neurobiol Lang (Camb). 2024. PMID: 38911464 Free PMC article.
References
- Adams C. D. (1982). Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q. J. Exp. Psychol. B 34, 77–98. 10.1080/14640748208400878
- Adams C. D., Dickinson A. (1981). Instrumental responding following reinforcer devaluation. Q. J. Exp. Psychol. B 33, 109–121. 10.1080/14640748108400816
- Colwill R. M., Rescorla R. A. (1985). Postconditioning devaluation of a reinforcer affects instrumental responding. J. Exp. Psychol. Anim. Behav. Process. 11, 120–132 10.1037//0097-7403.11.1.120 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous