Bacteria activate sensory neurons that modulate pain and inflammation - PubMed (original) (raw)
. 2013 Sep 5;501(7465):52-7.
doi: 10.1038/nature12479. Epub 2013 Aug 21.
Balthasar A Heesters, Nader Ghasemlou, Christian A Von Hehn, Fan Zhao, Johnathan Tran, Brian Wainger, Amanda Strominger, Sriya Muralidharan, Alexander R Horswill, Juliane Bubeck Wardenburg, Sun Wook Hwang, Michael C Carroll, Clifford J Woolf
Affiliations
- PMID: 23965627
- PMCID: PMC3773968
- DOI: 10.1038/nature12479
Bacteria activate sensory neurons that modulate pain and inflammation
Isaac M Chiu et al. Nature. 2013.
Abstract
Nociceptor sensory neurons are specialized to detect potentially damaging stimuli, protecting the organism by initiating the sensation of pain and eliciting defensive behaviours. Bacterial infections produce pain by unknown molecular mechanisms, although they are presumed to be secondary to immune activation. Here we demonstrate that bacteria directly activate nociceptors, and that the immune response mediated through TLR2, MyD88, T cells, B cells, and neutrophils and monocytes is not necessary for Staphylococcus aureus-induced pain in mice. Mechanical and thermal hyperalgesia in mice is correlated with live bacterial load rather than tissue swelling or immune activation. Bacteria induce calcium flux and action potentials in nociceptor neurons, in part via bacterial N-formylated peptides and the pore-forming toxin α-haemolysin, through distinct mechanisms. Specific ablation of Nav1.8-lineage neurons, which include nociceptors, abrogated pain during bacterial infection, but concurrently increased local immune infiltration and lymphadenopathy of the draining lymph node. Thus, bacterial pathogens produce pain by directly activating sensory neurons that modulate inflammation, an unsuspected role for the nervous system in host-pathogen interactions.
Figures
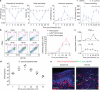
Figure 1. S. aureus infection induces pain hypersensitivity paralleling bacterial load but not immune activation
(a) S. aureus infection induces mechanical hypersensitivity (p=0.0021, n= 10/group), heat hypersensitivity (p<0.0001, n=10/group), acetone cold response (p<0.0001, n=20/group), and tissue swelling (p<0.0001, n=10/group). *p<0.05, ***p<0.001. (b) Left, flow cytometry shows myeloid (CD11b+CD45+) but not lymphoid (CD11b−CD45+) immune expansion in infected tissues. Right, Quantification of infected tissue neutrophils (CD11b+Ly6G+), Ly6chi monocytes (CD11b+Ly6G−Ly6Chi), and Ly6Clo monocytes/macrophages (CD11b+Ly6G−Ly6Clo). n=3/time-point. (c) TNF-α, IL-1β levels in infected tissues. n=4/time-point. (d) Bacterial load recovery. n=4/time-point. (e) GFP-S. aureus are in proximity with Nav1.8-Cre/TdTomato+ dermal nerve fibers, 3 hours post-infection. Scalebar,100 μm. Error bars, mean±s.e.m.
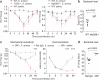
Figure 2. Innate immunity through TLR2/MyD88 and neutrophils/monocytes is not necessary for pain during S. aureus infection
(a) Infection-induced mechanical hypersensitivity is similar in TLR2−/− mice (n=10 infected) compared to WT mice (n=10 infected, n=10 saline injected) (p=0.744), and MyD88−/− mice (n=10 infected) relative to WT mice (n=11 infected, n=7 saline injected) (p=0.533). (b) Bacterial load, 3 days post-infection (n=5 each). (c) Infection-induced mechanical (p<0.0001) and heat (p<0.0001) hypersensitivity are increased in GR1 treated mice (n=10 infected, n=10 saline) compared to rat IgG treated mice (n=10 infected). Bonferroni, ***p<0.001. (d) Bacterial load, 2 days post-infection (n=6 each). Error bars, mean±s.e.m.
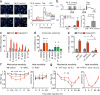
Figure 3. Bacterial heat-stable components including N-formylated peptides activate nociceptors
(a) Hk-S. aureus induces calcium flux in capsaicin, KCl responsive DRG neurons (arrows, traces). (b) (i) Representative recording, (ii) firing frequency upon hk-S. aureus application (5 capsaicin-responsive cells, 9 unresponsive). (c) DRG responsive proportions to hk-bacteria (n=4-26 fields/condition). (d) Acute pain induction: saline (n=13), hk-S. aureus (n=12), hk-S. pneumonia (n=14), hk-L. monocytogenes (n=5), hk-M. fermentans (n=6), hk-H. pylori (n=5), hk-P. aeruginosa (n=8), hk-E. coli (n=6). **p<0.01, *p<0.05. (e) DRG responsive proportions to formyl peptides (n=3-14 fields/condition). (f-g) fMLF, fMIFL injection induces mechanical hypersensitivity. Fpr1−/− mice show reduced hk-S. aureus mechanical hypersensitivity (p=0.0089). fMIFL vs. saline, Fpr1−/− vs. WT: *p<0.05; **p<0.01; ***p<0.001. Error bars, mean±s.e.m.
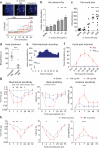
Figure 4. Heat-sensitive S. aureus Hla activates nociceptors and contributes to infection-induced hyperalgesia
(a) Hla application evoked DRG neuron calcium flux (arrows, traces), (b-c) dose-dependent calcium flux (n=3/condition) and acute pain (n=5-10/group). *p<0.05, ***p<0.001. (d) Heat pre-treatment abolishes Hla-induced pain (1 μg, n=7/group). (e) Hla, HLAH35L evoked DRG neuron action potentials (arrow, Hla application, n=3/condition). (f) Hla (1 μg, n=6) but not HlaH35L (1 μg, n=5) induced acute pain. (g) Hla 100 ng (n=8), 330 ng (n=8), saline (n=8) injection induced hypersensitivity. 100 ng vs. saline: ***p<0.001; 330 ng vs. saline: #p<0.05, ##p<0.01, ###p<0.001. (h) S. aureus lacking Hla (n=12) produced less mechanical (p=0.0056), heat (p=0.0193), acetone (p=0.0118) hypersensitivity than WT S. aureus (n=13). *p<0.05; **p<0.01; ***p<0.001. Error bars, mean±s.e.m.
Figure 5. Nociceptor ablation leads to increased local inflammation and lymphadenopathy following S. aureus infection
(a) Nav1.8-Cre/DTA neurons lack hk-bacteria responses. (b) Infection-induced mechanical (p=0.0027), heat hypersensitivity (p=0.0003) in Nav1.8-Cre/DTA mice (n=10 mechanical, n=6 heat) and Control littermates (n=12 mechanical, n=6 heat). ***p<0.001. (c-e) Parameters analyzed 24 hours post-infection. (c) Tissue swelling: Nav1.8-Cre/DTA (n=23), Control (n=19). (d) Plantar neutrophils/monocytes: Nav1.8-Cre/DTA: n=4 uninfected, n=15 infected; Control: n=4 uninfected, n=19 infected. (e) Popliteal lymph node images (infected), lymph node cellularity (Nav1.8-Cre/DTA: n=9 uninfected, n=10 infected; Control: n=9 uninfected, n=11 infected), and lymph node monocyte/macrophage (Mϕ), neutrophil (Nϕ), T, B cell subsets (n=5 each). Error bars, mean±s.e.m.
Figure 6. Nociceptor derived neuropeptides regulate innate immune activation
(a) Nav1.8-Cre/TdTomato+ DRG, trigeminal, nodose ganglia neurons were purified by flow cytometry (gates shown). (b) Top 30 nociceptor-expressed neuropeptides and myeloid immune cell-expressed neuropeptide receptors, shown from maximum to minimum. (c) Hla, S. aureus supernatant, and capsaicin (100 nM) induce DRG neuron CGRP release. **p<0.01; ***p<0.001. (d) TNF-α production by hk-S. aureus or Lipoteichoic acid stimulated macrophages was suppressed by CGRP, Sst, Gal (neuropeptide concentrations, 1 μM; *p<0.05). (e) CGRP injection decreased lymphadenopathy 24 hours post-S. aureus infection. Error bars, mean±s.e.m.
Comment in
- Neuroscience: Bacteria get on your nerves.
Nizet V, Yaksh T. Nizet V, et al. Nature. 2013 Sep 5;501(7465):43-4. doi: 10.1038/nature12550. Epub 2013 Aug 21. Nature. 2013. PMID: 23965630 No abstract available. - Neuroimmunology: pain--blame it on the bug, eh?
Bordon Y. Bordon Y. Nat Rev Immunol. 2013 Oct;13(10):706. doi: 10.1038/nri3533. Epub 2013 Sep 2. Nat Rev Immunol. 2013. PMID: 23995628 No abstract available.
Similar articles
- Bacteria and the neural code.
Steinberg BE, Tracey KJ, Slutsky AS. Steinberg BE, et al. N Engl J Med. 2014 Nov 27;371(22):2131-3. doi: 10.1056/NEJMcibr1412003. N Engl J Med. 2014. PMID: 25427116 No abstract available. - Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia.
Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM. Baral P, et al. Nat Med. 2018 May;24(4):417-426. doi: 10.1038/nm.4501. Epub 2018 Mar 5. Nat Med. 2018. PMID: 29505031 Free PMC article. - Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation.
Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, Paust S, Wood JN, von Andrian UH. Riol-Blanco L, et al. Nature. 2014 Jun 5;510(7503):157-61. doi: 10.1038/nature13199. Epub 2014 Apr 23. Nature. 2014. PMID: 24759321 Free PMC article. - Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation.
Pinho-Ribeiro FA, Verri WA Jr, Chiu IM. Pinho-Ribeiro FA, et al. Trends Immunol. 2017 Jan;38(1):5-19. doi: 10.1016/j.it.2016.10.001. Epub 2016 Oct 25. Trends Immunol. 2017. PMID: 27793571 Free PMC article. Review. - Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.
Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM. Guerra FE, et al. Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
Cited by
- Modulation of persistent bladder pain in mice: The role of macrophage migration inhibitory factor, high mobility group box-1, and downstream signaling pathways.
Ye S, Ma F, Mahmood DFD, Vera PL. Ye S, et al. Bladder (San Franc). 2024 Oct 28;11(2):e21200011. doi: 10.14440/bladder.2024.0015. eCollection 2024. Bladder (San Franc). 2024. PMID: 39539469 Free PMC article. - Inflammation is associated with pain and fatigue in older adults.
Norton SA, Blaydon LM, Niehaus M, Miller AP, Hill PL, Oltmanns TF, Bogdan R. Norton SA, et al. Brain Behav Immun Health. 2024 Oct 18;42:100874. doi: 10.1016/j.bbih.2024.100874. eCollection 2024 Dec. Brain Behav Immun Health. 2024. PMID: 39525304 Free PMC article. - The influence of sex on neuroimmune communication, pain, and physiology.
Alexander SN, Green AR, Debner EK, Ramos Freitas LE, Abdelhadi HMK, Szabo-Pardi TA, Burton MD. Alexander SN, et al. Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review. - Research progress in the clinical application of inhaled anesthetic sevoflurane.
Wu H, Wang S, Dai FB, Tang CL. Wu H, et al. Med Gas Res. 2025 Mar 1;15(1):85-92. doi: 10.4103/mgr.MEDGASRES-D-23-00003. Epub 2024 Oct 2. Med Gas Res. 2025. PMID: 39436171 Free PMC article. Review. - Neuropeptide signalling orchestrates T cell differentiation.
Hou Y, Sun L, LaFleur MW, Huang L, Lambden C, Thakore PI, Geiger-Schuller K, Kimura K, Yan L, Zang Y, Tang R, Shi J, Barilla R, Deng L, Subramanian A, Wallrapp A, Choi HS, Kye YC, Ashenberg O, Schiebinger G, Doench JG, Chiu IM, Regev A, Sharpe AH, Kuchroo VK. Hou Y, et al. Nature. 2024 Nov;635(8038):444-452. doi: 10.1038/s41586-024-08049-w. Epub 2024 Oct 16. Nature. 2024. PMID: 39415015
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. - PubMed
- White RJ. Wound infection-associated pain. J Wound Care. 2009;18:245–249. - PubMed
- Morgan M. Treatment of MRSA soft tissue infections: an overview. Injury. 2011;42(Suppl 5):S11–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI039246/AI/NIAID NIH HHS/United States
- R37 NS039518/NS/NINDS NIH HHS/United States
- R37NS039518/NS/NINDS NIH HHS/United States
- 5F32NS076297/NS/NINDS NIH HHS/United States
- P01 NS072040/NS/NINDS NIH HHS/United States
- F32 NS076297/NS/NINDS NIH HHS/United States
- P01AI078897/AI/NIAID NIH HHS/United States
- R01 NS039518/NS/NINDS NIH HHS/United States
- 5R01AI039246/AI/NIAID NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- P01 AI078897/AI/NIAID NIH HHS/United States
- P30-HD018655/HD/NICHD NIH HHS/United States
- 5P01NS072040/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases