Protrudin binds atlastins and endoplasmic reticulum-shaping proteins and regulates network formation - PubMed (original) (raw)
Protrudin binds atlastins and endoplasmic reticulum-shaping proteins and regulates network formation
Jaerak Chang et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Hereditary spastic paraplegias are inherited neurological disorders characterized by progressive lower-limb spasticity and weakness. Although more than 50 genetic loci are known [spastic gait (SPG)1 to -57], over half of hereditary spastic paraplegia cases are caused by pathogenic mutations in four genes encoding proteins that function in tubular endoplasmic reticulum (ER) network formation: atlastin-1 (SPG3A), spastin (SPG4), reticulon 2 (SPG12), and receptor expression-enhancing protein 1 (SPG31). Here, we show that the SPG33 protein protrudin contains hydrophobic, intramembrane hairpin domains, interacts with tubular ER proteins, and functions in ER morphogenesis by regulating the sheet-to-tubule balance and possibly the density of tubule interconnections. Protrudin also interacts with KIF5 and harbors a Rab-binding domain, a noncanonical FYVE (Fab-1, YGL023, Vps27, and EEA1) domain, and a two phenylalanines in an acidic tract (FFAT) domain and, thus, may also function in the distribution of ER tubules via ER contacts with the plasma membrane or other organelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Membrane topology of protrudin. (A) Myc-tagged protrudin or atlastin-1 were transfected into HEK293T cells as indicated. Homogenates (Homog) were resolved into soluble and membrane (Memb) fractions, and aliquots were immunoblotted (IB). Calnexin and β-tubulin are markers for membrane and cytosolic fractions, respectively. (B) Membrane fractions from cells expressing HA-protrudin or REEP1-HA were phase partitioned with Triton X-114 and then immunoblotted for HA-epitope or Grp78. (C) Protease protection assays. Microsomes from Myc-protrudin–expressing cells were treated with PK. Aliquots were immunoblotted with Myc-epitope (N terminus of protrudin) or anti-protrudin (C-terminal region of protrudin) antibodies. Calnexin (partly luminal) and Grp78 (completely luminal) were used to monitor proteolysis. A major proteolytic fragment of protrudin is denoted with an arrow. (D) Cells were transfected with wild-type (WT) Myc-protrudin or else Y127N and V149N mutants, and lysates were immunoblotted as shown. (E) Membrane topology model for protrudin. CC, coiled-coil; RBD, Rab-binding domain. Migrations of molecular-mass standards (in kilodaltons) are to the left in B_–_D.
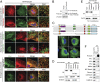
Fig. 2.
Protrudin localizes to the ER via its intramembrane domains and interacts with ER-shaping proteins. (A) From top to bottom, HeLa cells transiently expressing HA-protrudin were immunostained for endogenous REEP5 or calreticulin (red), along with HA-epitope (green). HeLa cells stably expressing HA-protrudin were immunostained for endogenous REEP5 or CLIMP-63 (green) and HA-epitope (red). Cells expressing Myc–atlastin-3 were coimmunostained for Myc-epitope (green) and endogenous protrudin (red). HeLa cells were costained for endogenous calnexin (green) and endogenous protrudin (red). Insets in the merged images (with DAPI staining) are enlarged to the right. (B) Lysates from cells stably expressing HA-protrudin were immunoblotted as shown. Endo., endogenous. (C, Upper) Schematic diagram of human protrudin and ΔTM deletion constructs. CC, coiled-coil; RBD, Rab-binding domain. (C, Lower) Cells expressing HA-tagged wild-type (WT) protrudin and the indicated deletion mutants were stained for HA-epitope (green) and DAPI (blue). (D) Wild-type and mutant protrudin proteins as in C were expressed in HEK293T cells. Homogenates (Homog) were separated into soluble and membrane (Memb) fractions and immunoblotted. (E) Membrane fractions from cells expressing wild-type and mutant protrudin proteins were phase partitioned with Triton X-114 and then immunoblotted for HA-epitope or Grp78. (F) Endogenous protrudin in HEK293T cells was immunoprecipitated (IP) with anti-protrudin antibodies or control IgG, and precipitates were immunoblotted as shown. ATL, atlastin; KTN, kinectin. (Scale bars: 10 μm.)
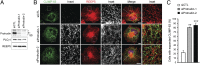
Fig. 3.
Protrudin plays a role in forming the tubular ER network. (A) Two different siRNAs against human protrudin were transfected into HeLa cells for 72 h, and cell lysates were immunoblotted. An asterisk (*) indicates a cross-reacting band, and an arrow identifies protrudin. Migrations of molecular-mass standards are to the right. (B) siRNA-transfected HeLa cells were immunostained for CLIMP-63 (green) and REEP5 (red). Insets are enlarged to the right of each image. (Scale bars: 10 μm.) Line-scan plots are shown in
Fig. S6_A_
. (C) Numbers of cells with expanded CLIMP-63 signal area were quantified in control and protrudin-depleted conditions (n = 3; >200 cells per experiment). Means ± SD are shown. Paired Student t test: **P < 0.01; ***P < 0.001.
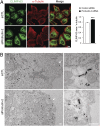
Fig. 4.
Protrudin depletion modulates the ER sheet-to-tubule balance. (A) HeLa cells were transfected with control (siCTL) (n = 33) or protrudin (n = 22) siRNAs for 72 h and then immunostained with CLIMP-63 (green) and α-tubulin (red). All studies were done at the same time, with the same procedures and microscope settings. Pixel areas of CLIMP-63 were normalized to those of α-tubulin using ImageJ tools. Data are presented in arbitrary units. Means ± SEM are shown. Student t test: ***P < 0.001. (Scale bar: 10 μm.) (B) siRNA-transfected HeLa cells were immunogold-labeled for a luminal epitope of CLIMP-63, with visualization by electron microscopy. N, nucleus. Arrowheads denote the plasma membrane. (Scale bars: 500 nm.)
Similar articles
- Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network.
Park SH, Zhu PP, Parker RL, Blackstone C. Park SH, et al. J Clin Invest. 2010 Apr;120(4):1097-110. doi: 10.1172/JCI40979. J Clin Invest. 2010. PMID: 20200447 Free PMC article. - Protrudin regulates endoplasmic reticulum morphology and function associated with the pathogenesis of hereditary spastic paraplegia.
Hashimoto Y, Shirane M, Matsuzaki F, Saita S, Ohnishi T, Nakayama KI. Hashimoto Y, et al. J Biol Chem. 2014 May 9;289(19):12946-61. doi: 10.1074/jbc.M113.528687. Epub 2014 Mar 25. J Biol Chem. 2014. PMID: 24668814 Free PMC article. - Mutations in the ER-shaping protein reticulon 2 cause the axon-degenerative disorder hereditary spastic paraplegia type 12.
Montenegro G, Rebelo AP, Connell J, Allison R, Babalini C, D'Aloia M, Montieri P, Schüle R, Ishiura H, Price J, Strickland A, Gonzalez MA, Baumbach-Reardon L, Deconinck T, Huang J, Bernardi G, Vance JM, Rogers MT, Tsuji S, De Jonghe P, Pericak-Vance MA, Schöls L, Orlacchio A, Reid E, Züchner S. Montenegro G, et al. J Clin Invest. 2012 Feb;122(2):538-44. doi: 10.1172/JCI60560. Epub 2012 Jan 9. J Clin Invest. 2012. PMID: 22232211 Free PMC article. - Hereditary spastic paraplegia SPG4: what is known and not known about the disease.
Solowska JM, Baas PW. Solowska JM, et al. Brain. 2015 Sep;138(Pt 9):2471-84. doi: 10.1093/brain/awv178. Epub 2015 Jun 20. Brain. 2015. PMID: 26094131 Free PMC article. Review. - Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms.
Fink JK. Fink JK. Acta Neuropathol. 2013 Sep;126(3):307-28. doi: 10.1007/s00401-013-1115-8. Epub 2013 Jul 30. Acta Neuropathol. 2013. PMID: 23897027 Free PMC article. Review.
Cited by
- ER-endosome contact sites: molecular compositions and functions.
Raiborg C, Wenzel EM, Stenmark H. Raiborg C, et al. EMBO J. 2015 Jul 14;34(14):1848-58. doi: 10.15252/embj.201591481. Epub 2015 Jun 3. EMBO J. 2015. PMID: 26041457 Free PMC article. Review. - Yeast lunapark regulates the formation of _trans_-Sey1p complexes for homotypic ER membrane fusion.
Jang E, Lee M, Yoon SY, Lee SS, Park J, Jin MS, Eom SH, Lee C, Jun Y. Jang E, et al. iScience. 2023 Nov 2;26(12):108386. doi: 10.1016/j.isci.2023.108386. eCollection 2023 Dec 15. iScience. 2023. PMID: 38025788 Free PMC article. - PAR3-PAR6-atypical PKC polarity complex proteins in neuronal polarization.
Hapak SM, Rothlin CV, Ghosh S. Hapak SM, et al. Cell Mol Life Sci. 2018 Aug;75(15):2735-2761. doi: 10.1007/s00018-018-2828-6. Epub 2018 Apr 25. Cell Mol Life Sci. 2018. PMID: 29696344 Free PMC article. Review. - Protrudin-deficient mice manifest depression-like behavior with abnormalities in activity, attention, and cued fear-conditioning.
Shirane M, Shoji H, Hashimoto Y, Katagiri H, Kobayashi S, Manabe T, Miyakawa T, Nakayama KI. Shirane M, et al. Mol Brain. 2020 Nov 10;13(1):146. doi: 10.1186/s13041-020-00693-3. Mol Brain. 2020. PMID: 33172474 Free PMC article. - Protrudin and PDZD8 contribute to neuronal integrity by promoting lipid extraction required for endosome maturation.
Shirane M, Wada M, Morita K, Hayashi N, Kunimatsu R, Matsumoto Y, Matsuzaki F, Nakatsumi H, Ohta K, Tamura Y, Nakayama KI. Shirane M, et al. Nat Commun. 2020 Sep 11;11(1):4576. doi: 10.1038/s41467-020-18413-9. Nat Commun. 2020. PMID: 32917905 Free PMC article.
References
- Schüle R, Schöls L. Genetics of hereditary spastic paraplegias. Semin Neurol. 2011;31(5):484–493. - PubMed
- Finsterer J, et al. Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance. J Neurol Sci. 2012;318(1-2):1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials