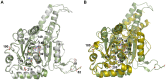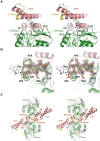Functional mapping of human dynamin-1-like GTPase domain based on x-ray structure analyses - PubMed (original) (raw)
Functional mapping of human dynamin-1-like GTPase domain based on x-ray structure analyses
Julia Wenger et al. PLoS One. 2013.
Abstract
Human dynamin-1-like protein (DNM1L) is a GTP-driven molecular machine that segregates mitochondria and peroxisomes. To obtain insights into its catalytic mechanism, we determined crystal structures of a construct comprising the GTPase domain and the bundle signaling element (BSE) in the nucleotide-free and GTP-analogue-bound states. The GTPase domain of DNM1L is structurally related to that of dynamin and binds the nucleotide 5'-Guanylyl-imidodiphosphate (GMP-PNP) via five highly conserved motifs, whereas the BSE folds into a pocket at the opposite side. Based on these structures, the GTPase center was systematically mapped by alanine mutagenesis and kinetic measurements. Thus, residues essential for the GTPase reaction were characterized, among them Lys38, Ser39 and Ser40 in the phosphate binding loop, Thr59 from switch I, Asp146 and Gly149 from switch II, Lys216 and Asp218 in the G4 element, as well as Asn246 in the G5 element. Also, mutated Glu81 and Glu82 in the unique 16-residue insertion of DNM1L influence the activity significantly. Mutations of Gln34, Ser35, and Asp190 in the predicted assembly interface interfered with dimerization of the GTPase domain induced by a transition state analogue and led to a loss of the lipid-stimulated GTPase activity. Our data point to related catalytic mechanisms of DNM1L and dynamin involving dimerization of their GTPase domains.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
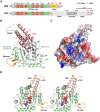
Figure 1. Overall structure of the DNM1L GTPase-GED fusion protein.
(A) Schematic representation of the construct expressed in E. coli and used for crystallization. The GTP binding stretches P-loop, switches I and II (S1 and S2), as well as G4 and G5 are depicted in red. The unique DNM1L insertion, denoted 80-loop, is shown in orange, the artificial (GS)4 shortcut in yellow and the bundle signaling element (BSE) in salmon. Also, the GTPase effector domain (GED) is indicated. Residue numbering follows the original human sequence of isoform 1 starting with Met1. The C-terminal linker with His6 tag is not included. (B) Tertiary structure of the nucleotide-free DNM1L GTPase-GED with secondary structural elements labels. The GTPase core homologous to human Ras is displayed in grey with dynamin-1-typical insertions in green and the BSE in salmon, the shortcut linker in yellow, and the 80-loop in orange. The conformation of the BSE represents the more compact closed or post-fission state of dynamin-like proteins. (C) Surface potential representation of the DNM1L GG structure with GMP-PNP shown as stick model bound in the active site cleft, turned around the y-axis by 180° with respect to Fig. 1B. The electrostatic potential at the molecular surface ranges from −120 to +120 kBT/e, with negatively charged regions depicted in red and positively charged ones in blue. (D) Stereo view of nucleotide-bound DNM1L GTPase-GED. GMP-PNP is depicted as atomic sphere model bound in the active site cleft with the nucleotide binding stretches colored in red. Otherwise, the color scheme is according to Fig. 1B, except for the whole GTPase domain shown in green. Atom colors are carbon in grey, oxygen in red, nitrogen in blue and phosphorus in orange. This nucleotide-free structure corresponds to the closed or post-fission state.
Figure 2. Close-up views of the active site cleft in the nucleotide-free and bound structures of the DNM1L GG construct in stereo.
(A) The nucleotide-free form with the most relevant residue side chains of the five GTP binding stretches and citrate (FLC, yellow) displayed as stick models. Electron density of a 2Fo-Fc map is shown in grey and contoured at 1σ. The red sphere designates the catalytic water (C). (B) GMP-PNP complex of the DNM1L GG construct. The nucleotide is shown as stick model, while the red spheres represent water molecules, such as the bridging water (B) and one, which binds to the α-phosphate. The electron density of a 2Fo-Fc map is shown in grey and contoured at 1σ, surrounding the nucleotide and relevant parts of the structure with significant conformational changes with respect to the nucleotide-free form.
Figure 3. Superposition of the two DNM1L GG structures and dynamin-1 GG.
(A) Overlay of the nucleotide-free DNM1L GG structure in white with the GMP-PNP-bound structure in green (shown without ligands). Side chains that were mutated in our study are shown as stick models with sequence number labels. (B) Overlay of dynamin-1 (PDB code 2X2F) in yellow with the structure of GMP-PNP-bound DNM1L in green. Mutated residues of DNM1L that are equivalent to those of dynamin (see Fig. 3A) are displayed as side chain stick models with dynamin sequence numbers (depicted without ligands).
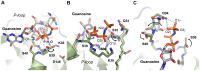
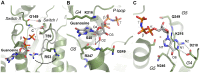
Figure 4. Detailed views of GTP-binding in the P-loop of DNM1L.
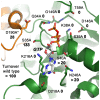
(A) Four hydrogen bonds fix the α-phosphate to the P-loop: O1α to Ser40 Oγ (distance 2.48 Å) and NH (3.45 Å), O3α to NHs of Ser39 (3.34 Å) and Lys38 (3.30 Å). Additionally, the O2α binds an H2O (3.39 Å). Lys38 is stabilized by a hydrogen bond to Asp146 from switch II. The overlaid apo-structure in grey shows that the Ser39 side chain rotates about 180° upon GTP binding, to a conformation (in green) that is suitable to stabilize a GTP transition state as seen in other structures of dynamins, e.g. in complex with GDP-AlF4 −. (B) Interactions that fix the β-phosphate: O1β forms hydrogen bonds to the NHs of Ser36 (3.31 Å) and of Ser35 (2.94 Å), while the O2β only binds the Lys38 NH (3.49 Å). The Nζ of Lys38 is more than 4 Å away from the O2β, but has the capacity to stabilize together with the Ser39 side chain the phosphate portion of the GTP, as seen in other dynamin-1 nucleotide analogue complexes. (C) The γ-phosphate forms hydrogen bonds via its O2γ to the Ser35 NH (3.48 Å) and to the Nε2 of Gln34 (3.14 Å), which also binds the O1γ (2.72 Å). The Gln34 side chain rotates significantly from the apo-conformation (light grey) to the nucleotide-conformation (green). The 180° peptide flip between Gln34-Ser35 brings Ser35 NH in a position suitable for O1β and O2γ binding, accompanied by a 180° side chain rotation of Ser35. Similar peptide flips occur in apo-nucleotide pairs of mammalian and D. discoideum dynamins.
Figure 5. Detailed views of the GTP-binding elements switch I, switch II, G4 and G5 of DNM1L.
(A) The canonical Mg2+ site between O1β and the O2γ is not occupied in the GMP-PNP-DNM1L structure (green). Also, no significant positional shift of Thr59 from the switch I loop takes place between apo- (grey) and nucleotide form (green). The unnatural N3β atom may favour the γ-phosphate conformation rotated by about 60° with respect to the transition state of GTP, shifting it about 2.5 Å away from the catalytic water. Only the nucleotide-free form exhibits the catalytic water molecule (C, grey) bound at the Thr59 carbonyl O (3.15 Å) and connected to switch II via the NH of Gly149 (2.87 Å). The bridging H2O (B, red) is only present in the nucleotide complex, bound to the Gly149 carbonyl O (3.21 Å). Upon GMP-PNP binding, the Arg53 side chain moves out of the active site, making room for an H2O, which binds O2 of the α-phosphate. (B) The ribose of GMP-PNP forms bonds with the ether oxygen O4 to the Nζ of Lys216 (3.16 Å), and with the hydroxyl group of O2 to an H2O (3.25 Å), which is bonded to the carbonyl O of Arg247 (2.45 Å) and the Ser40 Oγ (3.01 Å). Another bond is formed by the ribose O2 to Gln249 NH (2.79 Å). (C) The Lys216 side chain, depicted as thin stick model for clarity, covers the aromatic rings of the guanine part, while the Asp218 carboxylate binds the amino N2 (2.86 Å) and the N1 (3.12 Å). A further interaction from the Asn246 Oδ1 to the N7 (3.54 Å) might be mediated by an unresolved H2O, which could be bound to the carbonyl O of Gly37, as seen in other dynamin-nucleotide complexes.
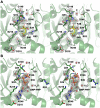
Figure 6. BSE-GTPase domain interface, 80-loop, and their interface at the 2-fold axis.
(A) The BSE-GTPase interface in the GMP-PNP complex. Both the nucleotide-free and –bound structures represent the closed or post-fission state of dynamin superfamily proteins and exhibit virtually no conformational differences. The interface of GTPase and BSE domain is characterized by mixed charged, polar, and hydrophobic interactions. (B) 80-loop insertion in the GTPase domain of DNM1L from Gln72 to Glu87. The insertion exhibits a short antiparallel β-sheet between Arg76 and Ala87. From Thr79 up to Gly84 the electron density of the 2Fo-Fc map, shown in grey and contoured at 1σ, is not well defined. (C) Polar interface between two symmetry-related DNM1L monomers A and A*. The 2-fold crystallographic axis (black oval) generates an interface that involves 14 hydrogen bonds and salt bridges of polar and charged side chains from the BSE and the 80-loop, together with roughly 20 water molecules (shown as sticks and red spheres, respectively). Both the GTPase domain and the stalks could form higher oligomers, while this dimer remains intact.
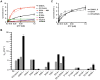
Figure 7. GTPase activity of DNM1L and the mutants.
(A) Basal GTPase activities of wild-type DNM1L, DNM1L GG fusion protein and full-length mutants. Steady-state GTPase activities of full-length wild-type DNM1L, GG fusion protein, active site mutants and predicted GTPase domain dimerization mutants (Q34A, S35A, D190A) were measured as described in the Methods section. Amino acid substitutions Q34A, K38A, S39A, T59A, D146A, G149A, K216A and D218A completely abolished GTP hydrolysis. The Q34A mutant is shown as one representative example for the inactive mutants. Among all these mutants, only S35A, S40A, D190A and N246A exhibited significant GTPase turnover. For S35A, both the simple Michaelis-Menten equation fit (label MM, orange dots) and the curve using a cooperative model (continuous orange line) with a Hill coefficient of 2.2 are depicted. Data are means of at least three independent experiments ± standard deviation (displayed as error bars) evaluated by nonlinear regression analysis. (B) Liposome-stimulated GTP hydrolysis of DNM1L and its mutants determined by multiple-turnover assays. Reactions were performed for 12 min at 37°C in the absence (grey bars) or presence (black bars) of PS liposomes. Initial hydrolysis rates kobs were determined by applying a linear fit to the data, with bars representing mean value ± standard deviation of three independent experiments. For mutants Q34A, S39A, T59A, D146A, G149A, and K216A less then 4% of the GTP was hydrolyzed within 12 minutes. (C) Basal GTP activity of full length DNM1L and the two loop mutants E81A and E81A/E82A. Although the three variants exhibit similar Michaelis-Menten curves, both mutants displayed lower Vmax (kobs) and faster saturation with GTP compared to WT.
Figure 8. GTPase domain interface model of the DNM1L GG fusion protein and nucleotide-dependent dimerization.
(A) Two chains of DNM1L molecules were superimposed on the GTPase domain dimer of _At_Drp1A (PDB code 3T34) as molecules A (green) and B (orange). The interface connecting residues Gln34, Ser35, Asp190, and GTP are depicted as stick models. In addition, the movement of the BSE domains between the pre- and postfission states is represented by the extended _At_Drp1A dimer (white) and the compact DNM1L dimer. The tetramer model (bottom, left) is based on full-length dynamin-1, which may further oligomerize via the stalks and other GTPase domains (green, orange). (B) Close-up view of the interface at Asp190 from molecule B and Gln34, Ser35 and GTP from molecule A. The conformations of the nucleotide-free and GMP-PNP bound structures are displayed. (C) Dimerization ability of the DNM1L GG fusion protein in the presence of different nucleotides. The GG fusion protein (60 µM) was subjected to gel filtration after incubation with different guanine nucleotide analogs (2 mM). Protein standards at 29 and 75 kDa are indicated. The dimeric protein eluted at a retention volume of 9.5 ml and monomeric protein at 11 ml. (D) SDS PAGE analysis of the SEC runs. Lane 1 shows purified GG fusion protein (41 kDa) followed by a molecular weight protein ladder (from top to bottom: 55 kDa, 43 kDa, 34 kDa). Elution volumes are indicated above. (E) Analysis of the DNM1L GMP-PNP complex stability under SEC conditions as in Fig. 8C. SEC elution (red) and further analysis of the peaks by HPLC (blue), with the indicated controls. (F) SEC of GG fusion protein mutants Q34A, S35A and D190A under conditions as in Fig. 8C in the presence of GDP⋅AlF4 −. Retention volumes of molecular weight standards are shown above.
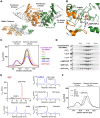
Figure 9. Structure-function map of the modelled DNM1L active site dimer.
All active site and dimerization residues that have been mutated to alanine are represented as stick models, as well as the GTP. The turnover numbers of the respective mutants as determined by the GTPase assay for basal activity are shown, whereby the WT was defined as 100%. Molecule A of the dimer is depicted in green, while the second molecule B is shown in orange, with the corresponding D190A*.
Figure 10. Conserved sequence motifs of the dynamin superfamily.
Sequence alignments of representative members of the dynamin superfamily comparing conserved GTP binding motifs and the _trans_-stabilizing loops important for G dimerization. Conserved key residues that were mutated to alanine in our study are marked with a dot. Species abbreviations are hs, Homo sapiens; rn, Rattus norvegicus; sc, Saccharomyces cerevisiae; dd, Dictyostelium discoideum; at, Arabidopsis thaliana; MxA, interferon-induced GTP-binding protein A; ! represents one of the amino acids of IV; # represents one of the amino acids NDQE.
Similar articles
- Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein.
Fröhlich C, Grabiger S, Schwefel D, Faelber K, Rosenbaum E, Mears J, Rocks O, Daumke O. Fröhlich C, et al. EMBO J. 2013 May 2;32(9):1280-92. doi: 10.1038/emboj.2013.74. Epub 2013 Apr 12. EMBO J. 2013. PMID: 23584531 Free PMC article. - Steric interference from intrinsically disordered regions controls dynamin-related protein 1 self-assembly during mitochondrial fission.
Lu B, Kennedy B, Clinton RW, Wang EJ, McHugh D, Stepanyants N, Macdonald PJ, Mears JA, Qi X, Ramachandran R. Lu B, et al. Sci Rep. 2018 Jul 18;8(1):10879. doi: 10.1038/s41598-018-29001-9. Sci Rep. 2018. PMID: 30022112 Free PMC article. - The mechanoenzymatic core of dynamin-related protein 1 comprises the minimal machinery required for membrane constriction.
Francy CA, Alvarez FJ, Zhou L, Ramachandran R, Mears JA. Francy CA, et al. J Biol Chem. 2015 May 1;290(18):11692-703. doi: 10.1074/jbc.M114.610881. Epub 2015 Mar 13. J Biol Chem. 2015. PMID: 25770210 Free PMC article. - Invited review: Mechanisms of GTP hydrolysis and conformational transitions in the dynamin superfamily.
Daumke O, Praefcke GJ. Daumke O, et al. Biopolymers. 2016 Aug;105(8):580-93. doi: 10.1002/bip.22855. Biopolymers. 2016. PMID: 27062152 Free PMC article. Review. - Dynamin GTPase, a force-generating molecular switch.
Warnock DE, Schmid SL. Warnock DE, et al. Bioessays. 1996 Nov;18(11):885-93. doi: 10.1002/bies.950181107. Bioessays. 1996. PMID: 8939066 Review.
Cited by
- Structural Insights into the Mechanism of Dynamin Superfamily Proteins.
Jimah JR, Hinshaw JE. Jimah JR, et al. Trends Cell Biol. 2019 Mar;29(3):257-273. doi: 10.1016/j.tcb.2018.11.003. Epub 2018 Dec 5. Trends Cell Biol. 2019. PMID: 30527453 Free PMC article. Review. - Hepatoprotective Role of Carvedilol against Ischemic Hepatitis Associated with Acute Heart Failure via Targeting miRNA-17 and Mitochondrial Dynamics-Related Proteins: An In Vivo and In Silico Study.
Mohamed DI, Ezzat SF, Elayat WM, El-Kharashi OA, El-Kareem HFA, Nahas HHA, Abdel-Wahab BA, Alshawwa SZ, Saleh A, Helmy YA, Khairy E, Saied EM. Mohamed DI, et al. Pharmaceuticals (Basel). 2022 Jul 5;15(7):832. doi: 10.3390/ph15070832. Pharmaceuticals (Basel). 2022. PMID: 35890131 Free PMC article. - Insights from Drosophila on Aβ- and tau-induced mitochondrial dysfunction: mechanisms and tools.
Varte V, Munkelwitz JW, Rincon-Limas DE. Varte V, et al. Front Neurosci. 2023 Apr 17;17:1184080. doi: 10.3389/fnins.2023.1184080. eCollection 2023. Front Neurosci. 2023. PMID: 37139514 Free PMC article. Review. - Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases.
Li RL, Wang LY, Duan HX, Zhang Q, Guo X, Wu C, Peng W. Li RL, et al. Front Pharmacol. 2022 Sep 22;13:937289. doi: 10.3389/fphar.2022.937289. eCollection 2022. Front Pharmacol. 2022. PMID: 36210852 Free PMC article. Review. - Multifaceted functions of Drp1 in hypoxia/ischemia-induced mitochondrial quality imbalance: from regulatory mechanism to targeted therapeutic strategy.
Hao S, Huang H, Ma RY, Zeng X, Duan CY. Hao S, et al. Mil Med Res. 2023 Oct 13;10(1):46. doi: 10.1186/s40779-023-00482-8. Mil Med Res. 2023. PMID: 37833768 Free PMC article. Review.
References
- Praefcke GJK, McMahon HT (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5: 133–147. - PubMed
- Saraste M, Sibbald PR, Wittinghofer A (1990) The P-loop – a common motif in ATP- and GTP-binding proteins. Trends in Biochemical Sciences 15: 430–434. - PubMed
- Vetter IR, Wittinghofer A (2001) The Guanine Nucleotide-Binding Switch in Three Dimensions. Science 294: 1299–1304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous