TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells - PubMed (original) (raw)
. 2013 Aug 16;8(8):e73424.
doi: 10.1371/journal.pone.0073424. eCollection 2013.
Wen-Feng Chu, Binlin Song, Monika Gooz, Jia-Ning Zhang, Chang-Jiang Yu, Shuai Jiang, Aleksander Baldys, Pal Gooz, Stacy Steele, Grzegorz Owsianik, Bernd Nilius, Peter Komlosi, P Darwin Bell
Affiliations
- PMID: 23977387
- PMCID: PMC3745395
- DOI: 10.1371/journal.pone.0073424
TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells
Zhi-Ren Zhang et al. PLoS One. 2013.
Abstract
Objective: Regulation of apical calcium entry is important for the function of principal cells of the collecting duct. However, the molecular identity and the regulators of the transporter/channel, which is responsible for apical calcium entry and what factors regulate the calcium conduction remain unclear.
Methods and results: We report that endogenous TRPP2 and TRPV4 assemble to form a 23-pS divalent cation-permeable non-selective ion channel at the apical membrane of renal principal cells of the collecting duct. TRPP2\TRPV4 channel complex was identified by patch-clamp, immunofluorescence and co-immunprecipitation studies in both principal cells that either possess normal cilia (cilia (+)) or in which cilia are absent (cilia (-)). This channel has distinct biophysical and pharmacological and regulatory profiles compared to either TRPP2 or TRPV4 channels. The rate of occurrence detected by patch clamp was higher in cilia (-) compared to cilia (+) cells. In addition, shRNA knockdown of TRPP2 increased the prevalence of TRPV4 channel activity while knockdown of TRPV4 resulted in TRPP2 activity and knockdown of both proteins vastly decreased the 23-pS channel activity. Epidermal growth factor (EGF) stimulated TRPP2\TRPV4 channel through the EGF receptor (EGFR) tyrosine kinase-dependent signaling. With loss of cilia, apical EGF treatment resulted in 64-fold increase in channel activity in cilia (-) but not cilia (+) cells. In addition EGF increased cell proliferation in cilia (-) cell that was dependent upon TRPP2\TRPV4 channel mediated increase in intracellular calcium.
Conclusion: We conclude that in the absence of cilia, an EGF activated TRPP2\TRPV4 channel may play an important role in increased cell proliferation and cystogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
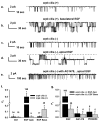
Figure 1. EGF regulates an apical 23-pS cation channel in both cilia (+) and cilia (-) cells.
(a–e) The representative single-channel traces were recorded from cilia (+) and cilia (-) cell using excised inside-out patch mode, respectively. 10 ng/mL EGF was added to the apical or the basolateral side for 5 to 10 min before recordings. The dashed gray lines indicate closed levels of current. Representative single-channel traces were recorded from cilia (+) (a & b) and cilia (-) cells (c & d) in the presence of basolateral or apical EGF, respectively. (e) A representative single-channel current was recorded from cilia (-) cell that was pretreated with 100 nM AG1478 for 30 min followed by apical administration of EGF. (f) Summary of P O values obtained under different experimental conditions in both cell types. * indicates significant difference from controls and # indicates significant difference between two cell types under control conditions; where Ctrl, EGF Apic and EGF Baso represent control, EFG applied at the apical and at the basolateral membrane, respectively. (g) Summary of the effects of tyrosine kinase inhibitor and MAPK inhibitor on EGF stimulated channel activity. * indicates the channel activity was significantly blunted by tyrosine kinase and MAPK inhibitors and # indicates EGF-induced channel activity is significantly different between the two cell types. Inserted numbers in Figure 1f & 1g represent n recordings under indicated experimental conditions.
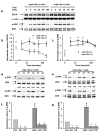
Figure 2. Cilia (-) cells has a much higher activity of EGFR and p-ERK than cilia (+) cells.
(a) The representative western blots showing the expression of EGFR, ERK, and p-ERK before and after 10 ng/mL EGF treatment from both apical and basolateral sides in both cell types for the indicated time. (b) Summary of EGFR expression levels, all data points were normalized to the value obtained from cilia (-) cells under control condition, i.e. no EGF simulation (n = 5 for each data point). (c) Summary of p-ERK expression levels, all data points were normalized to the value obtained from cilia (-) cells under control condition, i.e. no EGF simulation (n = 6 for each data point). * indicates p-ERK expression level of cilia (-) cells is significantly greater than that of cilia (+) cells. (d & e) The representative western blots showing the expression ERK and p-ERK with and without stimulation by 10 ng/mL EGF from both apical and basolateral sides in both cell types for 10 min, in the presence of variety of inhibitors. Where Crtl indicates control and AG, GF, GO, PD, and SB represent AG1478, GF109203, GO6976, PD98059, and SB202190, respectively. Cells were preincubated with EGF for 60 min, respectively. (f & g) Summary of p-ERK expression levels, all data points were normalized to the value obtained under control condition, i.e. no EGF simulation (n = 5 for each data point). * indicates the values were dramatically less than those of control and EGF stimulation. # indicates p-ERK expression level is significantly greater compared to control.
Figure 3. ~23-pS channel permeates divalent cation and mediates EGF-induced Ca2+ influx.
Cilia (-) cells were pretreated with 10 ng/mL EGF at the apical membrane prior to recordings. (a–c) The representative traces were recorded using excised inside-out patch mode at VM = 100 mV from cilia (-) cells and the dashed gray lines indicate closed current level. (a`–**c**`) The gray dashed lines indicate the fitted single-channel conductance (23.4 ± 0.76 pS) of the currents recorded under control condition (representative single-channel traces are shown in Figure S1; n = 13-21 for different data points). (a & a`) This representative recording contained at least four active channels. While the single-channel conductance of inward currents remained the same as control the single-channel conductance of outward currents was dramatically reduced to 2.79 ± 0.33 pS (n = 9-14 for different data points) by substitution of intracellular Na+ with equimolar NMDG+ and the reversal potential shifted to depolarization potentials (the red arrow head). (**b** & **b**`) The representative trace was recorded in the presence of equimolar intracellular K+ to extracellular Na+, which affected neither the single-channel conductance (the blue dashed line) nor the reversal potential (the blue arrow head). (c & c`) The representative trace was recorded in the presence of equimolar intracellular Ba2+ to extracellular Na+. Replacement of Na+ by Ba2+ did not alter the single-channel conductance of inward current, but altered the single-channel conductance of outward current (the green dashed line) and the reversal potential of the currents (where the green arrow head indicates the reversal potential that was corrected for the junction potential). (d & e) Manganese quenching assays demonstrate that EGF promotes Ca2+ entry in cilia (-) cells, but not in cilia (+) cells (data not shown); while double knockdown of TRPP2\TRPV4 channels or TRPV4, the effect of EGF on Ca2+ entry was abolished (n = 5); while knock down of TRPV4, EGF also promotes Ca2+ entry, albeit with less extent compared to wild-type cilia (-) cells (n = 5). * indicates significant difference compared to TRPV4 knocked down cilia (-) cells while stimulated with EGF.
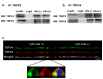
Figure 4. Co-IP and immunofluorescence staining demonstrating formation of a TRPP2\TRPV4 complex.
(a & b) Cells were immunoprecipitated with anti-TRPP2 and anti-TRPV4 antibodies, respectively. Co-precipitated complexes were probed by TRPV4 and TRPP2 antibodies, respectively. Cell lysates and n-IgG were served as controls. (c) Representative reconstructed side view (xz) immunofluorescence images of cilia (+) and cilia (-) cell monolayers fixed and labeled with antibodies raised against TRPV4 (green) and TRPP2 (red). Yellow color on the merged pseudocolor images at the bottom indicates colocalization of TRPV4 and TRPP2. The length of the bar in the bottom right is 30 µm. The inset demonstrates a single cell with a prominent apical cilium. Although TRPP2 appears to be more abundant at the tip of cilium, this is the result of a shift in the position of the red and green images in the z plane due to a slight chromatic aberration correction error in the z-plane.
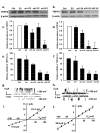
Figure 5. Effect of gene silencing on the 23-pS single-channel currents.
(a–b) Western blots showing knockdown of TRPP2 (left) and TRPV4 (right) by TRPP2- and TRPV4-specific shRNAs. (c–f) Summary of the data obtained from western blots (c & d; n = 5) and quantitative real-time PCR analysis (e & f; n = 3). Ctrl, EV, sh1, sh2 and sh3 represent non-tranfected (control), empty vector, shRNA1, shRNA2 and shRNA3 transfected cells, respectively. P2 and V4 indicate that the shRNAs were specific to TRPP2 and TRPV4, respectively. * indicates significant difference from control and empty vector transfected group. (g & h) Sample single-channel traces were recorded from cilia (-) cells expressing shRNA3s specific to TRPP2 and TRPV4, using excised inside-out patch configuration. (i & j) Summary of i-V plots constructed from the single-channel recordings as shown (g) and (h) demonstrating altered biophysical properties of the channels. Knockdown TRPP2 and TRPV4 resulted in the appearance of TRPV4-like channels (i) with a single-channel conductance of 115.7 ± 1.98 pS for outward and 51.8 ± 0.91 pS for inward currents, respectively and TRPP2-like currents with a single-channel conductance of 86.8 ± 1.78 pS (n = 5-9 for different data points).
Figure 6. EGF-induced activation of TRPP2\TRPV4 channels mediates hyperproliferation of cilia (-) cells.
(a) Plot shows the rate of cell proliferation. Under control conditions, cell proliferation rate was significantly higher in cilia (-) cells (the red dashed line) than in cilia (+) cells (the red solid line). EGF significantly enhanced the rate of cell proliferation in cilia (-) cells but not in cilia (+) cells. The effect of EGF on cell proliferation was abolished by AG1478, PD98059 and BAPTA in cilia (-) cells (n = 5 for each condition). (b & c) Western blots confirm the effectiveness of knockdown of both TRPP2 and TRPV4, where Ctrl, EV, sh3-P2\sh3-V4 represent non-tranfected (control), cells co-transfectd with empty vector, sh3-P2\sh3-V4 specific to TRPP2 and TRPV4, respectively. (d & e) Summary of the results obtained from western blots analysis; both TRPP2 and TRPV4 protein expression levels were reduced by ~75-80%. (f) Cell proliferation assays were performed at 96 hour time point after seeding the cells in the presence or in the absence of EGF. Plot shows that the rate of EGF-induced proliferation in cilia (-) cells was significantly reduced by knockdown TRPP2\TRPV4 channels. KO indicates knockdown, * indicates that under control condition the rate of cell proliferation was significantly higher in the presence of EGF. # indicates a significant difference compared to control.
Similar articles
- Loss of primary cilia increases polycystin-2 and TRPV4 and the appearance of a nonselective cation channel in the mouse cortical collecting duct.
Saigusa T, Yue Q, Bunni MA, Bell PD, Eaton DC. Saigusa T, et al. Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F632-F637. doi: 10.1152/ajprenal.00210.2019. Epub 2019 Jul 17. Am J Physiol Renal Physiol. 2019. PMID: 31313950 Free PMC article. - TRPP2 and TRPV4 form a polymodal sensory channel complex.
Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, Nilius B, Kuehn EW, Walz G. Köttgen M, et al. J Cell Biol. 2008 Aug 11;182(3):437-47. doi: 10.1083/jcb.200805124. J Cell Biol. 2008. PMID: 18695040 Free PMC article. - Dynamic coupling between TRPV4 and Ca2+-activated SK1/3 and IK1 K+ channels plays a critical role in regulating the K+-secretory BK channel in kidney collecting duct cells.
Li Y, Hu H, Tian JB, Zhu MX, O'Neil RG. Li Y, et al. Am J Physiol Renal Physiol. 2017 Jun 1;312(6):F1081-F1089. doi: 10.1152/ajprenal.00037.2017. Epub 2017 Mar 8. Am J Physiol Renal Physiol. 2017. PMID: 28274924 Free PMC article. - TRPP2 ion channels: The roles in various subcellular locations.
Tian PF, Sun MM, Hu XY, Du J, He W. Tian PF, et al. Biochimie. 2022 Oct;201:116-127. doi: 10.1016/j.biochi.2022.06.010. Epub 2022 Jun 26. Biochimie. 2022. PMID: 35760123 Review. - TRPP2 ion channels: Critical regulators of organ morphogenesis in health and disease.
Busch T, Köttgen M, Hofherr A. Busch T, et al. Cell Calcium. 2017 Sep;66:25-32. doi: 10.1016/j.ceca.2017.05.005. Epub 2017 Jun 1. Cell Calcium. 2017. PMID: 28807147 Review.
Cited by
- Molecular pathways and therapies in autosomal-dominant polycystic kidney disease.
Saigusa T, Bell PD. Saigusa T, et al. Physiology (Bethesda). 2015 May;30(3):195-207. doi: 10.1152/physiol.00032.2014. Physiology (Bethesda). 2015. PMID: 25933820 Free PMC article. Review. - A Novel Role for Polycystin-2 (Pkd2) in P. tetraurelia as a Probable Mg2+ Channel Necessary for Mg2+-Induced Behavior.
Valentine MS, Yano J, Van Houten J. Valentine MS, et al. Genes (Basel). 2019 Jun 14;10(6):455. doi: 10.3390/genes10060455. Genes (Basel). 2019. PMID: 31207979 Free PMC article. - Cholesterol Stimulates the Transient Receptor Potential Melastatin 4 Channel in mpkCCDc14 Cells.
Cai YX, Zhang BL, Yu M, Yang YC, Ao X, Zhu D, Wang QS, Lou J, Liang C, Tang LL, Wu MM, Zhang ZR, Ma HP. Cai YX, et al. Front Pharmacol. 2021 May 14;12:627875. doi: 10.3389/fphar.2021.627875. eCollection 2021. Front Pharmacol. 2021. PMID: 34054517 Free PMC article. - Functions of TRPs in retinal tissue in physiological and pathological conditions.
do Nascimento THO, Pereira-Figueiredo D, Veroneze L, Nascimento AA, De Logu F, Nassini R, Campello-Costa P, Faria-Melibeu ADC, Souza Monteiro de Araújo D, Calaza KC. do Nascimento THO, et al. Front Mol Neurosci. 2024 Sep 25;17:1459083. doi: 10.3389/fnmol.2024.1459083. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39386050 Free PMC article. Review. - The cAMP Signaling Pathway and Direct Protein Kinase A Phosphorylation Regulate Polycystin-2 (TRPP2) Channel Function.
Cantero Mdel R, Velázquez IF, Streets AJ, Ong AC, Cantiello HF. Cantero Mdel R, et al. J Biol Chem. 2015 Sep 25;290(39):23888-96. doi: 10.1074/jbc.M115.661082. Epub 2015 Aug 12. J Biol Chem. 2015. PMID: 26269590 Free PMC article.
References
- González-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E et al. (2001) Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci U S A 98: 1182-1187. doi:10.1073/pnas.98.3.1182. PubMed: 11252306. - DOI - PMC - PubMed
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B et al. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339-1342. doi:10.1126/science.272.5266.1339. PubMed: 8650545. - DOI - PubMed
- Bai CX, Giamarchi A, Rodat-Despoix L, Padilla F, Downs T et al. (2008) Formation of a new receptor-operated channel by heteromeric assembly of TRPP2 and TRPC1 subunits. EMBO Rep 9: 472-479. doi:10.1038/embor.2008.29. PubMed: 18323855. - DOI - PMC - PubMed
- Zhang P, Luo Y, Chasan B, González-Perrett S, Montalbetti N et al. (2009) The multimeric structure of polycystin-2 (TRPP2): structural-functional correlates of homo- and hetero-multimers with TRPC1. Hum Mol Genet 18: 1238-1251. doi:10.1093/hmg/ddp024. PubMed: 19193631. - DOI - PMC - PubMed
- Kobori T, Smith GD, Sandford R, Edwardson JM (2009) The transient receptor potential channels TRPP2 and TRPC1 form a heterotetramer with a 2:2 stoichiometry and an alternating subunit arrangement. J Biol Chem 284: 35507-35513. doi:10.1074/jbc.M109.060228. PubMed: 19850920. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous