Inhibition of the first step in synthesis of the mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45 - PubMed (original) (raw)
Inhibition of the first step in synthesis of the mycobacterial cell wall core, catalyzed by the GlcNAc-1-phosphate transferase WecA, by the novel caprazamycin derivative CPZEN-45
Yoshimasa Ishizaki et al. J Biol Chem. 2013.
Abstract
Because tuberculosis is one of the most prevalent and serious infections, countermeasures against it are urgently required. We isolated the antitubercular agents caprazamycins from the culture of an actinomycete strain and created CPZEN-45 as the most promising derivative of the caprazamycins. Herein, we describe the mode of action of CPZEN-45 first against Bacillus subtilis. Unlike the caprazamycins, CPZEN-45 strongly inhibited incorporation of radiolabeled glycerol into growing cultures and showed antibacterial activity against caprazamycin-resistant strains, including a strain overexpressing translocase-I (MraY, involved in the biosynthesis of peptidoglycan), the target of the caprazamycins. By contrast, CPZEN-45 was not effective against a strain overexpressing undecaprenyl-phosphate-GlcNAc-1-phosphate transferase (TagO, involved in the biosynthesis of teichoic acid), and a mutation was found in the tagO gene of the spontaneous CPZEN-45-resistant strain. This suggested that the primary target of CPZEN-45 in B. subtilis is TagO, which is a different target from that of the parent caprazamycins. This suggestion was confirmed by evaluation of the activities of these enzymes. Finally, we showed that CPZEN-45 was effective against WecA (Rv1302, also called Rfe) of Mycobacterium tuberculosis, the ortholog of TagO and involved in the biosynthesis of the mycolylarabinogalactan of the cell wall of M. tuberculosis. The outlook for WecA as a promising target for the development of antituberculous drugs as a countermeasure of drug resistant tuberculosis is discussed.
Keywords: Antibiotics Action; Bacillus; CPZEN-45; Caprazamycin B; Cell Wall; Mycolylarabinogalactan; TagO; Teichoic Acid; Tuberculosis; WecA.
Figures
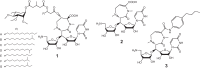
FIGURE 1.
Structures of caprazamycin A-G (1); caprazene (2), which is a product of the acid hydrolysate of caprazamycins; and CPZEN-45 (3), which is 4-butylanilide of caprazene.
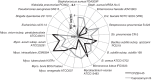
FIGURE 2.
Radar chart of antibacterial activity of caprazamycin B (gray line) and CPZEN-45 (black line).
FIGURE 3.
Strategy for the construction of the M. smegmatis mc2155 derivative strain, containing the WecA of M. tuberculosis expressed from the multicopy plasmid instead of the original. The PCR fragment of wecA of M. smegmatis (wecAMsm) was amplified using the genomic DNA of the mc2155 strain as the template. The consequent DNA fragment was digested with restriction enzymes Age I and Ngo_M IV, and the 5′-end and 3′-end fragments were ligated to induce deletion from nucleotide positions 46–612 of wecAMsm. This fragment (Δ_wecAMsm) was inserted into a plasmid containing the hygromycin-resistant gene (hygR), the levansucrase gene (sacB), and pUC replicon, and the resulting plasmid, pUChphsacB-Δ_wecAMsm_, was introduced into M. smegmatis mc2155 by electroporation. Because pUChphsacB-Δ_wecAMsm_ does not have a replication origin for mycobacteria, only when the plasmid was integrated into the genome did the transformants show resistance to hygromycin. The resulting strain was transformed with another plasmid, p16Rkan-wecAMtb, by electroporation. This plasmid was constructed as follows: the PCR fragment of wecA of the BCG strain of M. bovis (wecAMbo) was amplified using the genomic DNA of BCG as the template. This fragment was cloned into a plasmid containing the kanamycin-resistant gene (kanR), the pAL5000 replicon for mycobacteria, and the pUC replicon. The resulting plasmid was used as template of another PCR to alter wecAMbo to wecA of M. tuberculosis (wecAMtb), which has 1 base difference from wecAMbo. Finally, the resulting strain, which is resistant to hygromycin and kanamycin, was challenged on agar plates containing 10% sucrose. Because the levansucrase encoded by the sacB gene leads to sucrose sensitivity, when integrated plasmid pUChphsacB-Δ_wecAMsm_ was excluded from the genome, the transformant became sucrose-resistant again. The resulting sucrose-resistant and hygromycin-sensitive transformants had wild-type or disrupted wecA; thus, the genotype of each cell was analyzed by Southern blotting and transcription analysis using RT-PCR and subsequent sequencing.
FIGURE 4.
Effects of caprazamycin B, CPZEN-45, vancomycin, and tunicamycin on the incorporation of radiolabeled precursors into the macromolecules of B. subtilis 168. The percentage of the incorporation of [_methyl_-3H]thymidine (for DNA, squares), [5,6-3H]uridine (for RNA, diamonds),
l
-[4,5-3H]leucine (for protein, triangles), [1-14C]acetate (for fatty acids, crosses), _N_-acetyl-
d
-[1-14C]glucosamine (for peptidoglycan, closed circles), and [U-14C]glycerol (for teichoic acid, open circles) are shown. Each plot and error bars represent the average and standard deviation of three independent trials.
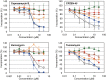
FIGURE 5.
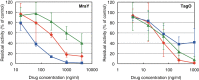
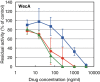
Effects of caprazamycin B, CPZEN-45, and tunicamycin on the activities of MraY and TagO in B. subtilis 168. The inhibitory curves of CPZEN-45 (diamonds), caprazamycin B (squares), and tunicamycin (triangles) on the activity of MraY and TagO are presented. Each plot and error bars represent the average and standard deviation of three independent trials.
FIGURE 6.
Effects of caprazamycin B, CPZEN-45, and tunicamycin on the activities of WecA of M. tuberculosis expressed in M. smegmatis 8a10. The inhibitory curves of CPZEN-45 (diamonds), caprazamycin B (squares), and tunicamycin (triangles) on the activity are presented. Each plot and error bars represent the average and standard deviation of three independent trials.
FIGURE 7.
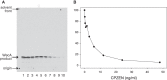
Effect of CPZEN-45 on the WecA activity of cytoplasmic membranes of M. smegmatis mc2155. A, TLC, developed as described above and subjected to radioautography, shows the effects of CPZEN-45 on the synthesis of the immediate product of WecA, the polyprenyl-P-P-GlcNAc. Preparation of membranes, cell-free assay conditions, including use of UDP-[U-14C]GlcNAc, were as described (22). B, CPZEN-45 was calculated to inhibit WecA of M. smegmatis strongly with an IC50 of 4.4 ng/ml. Data shown here are typical of three independent trials.
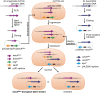
FIGURE 8.
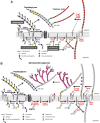
Overview of the biosynthetic pathway of peptidoglycan and teichoic acid in B. subtilis 168 (modified from Ref. 13) (A) and the biosynthetic pathway of peptidoglycan and mycolylarabinogalactan in M. tuberculosis (B). MurX and WecA are the orthologs of MraY and TagO in B. subtilis, respectively.
Similar articles
- Synthesis and biological activity of analogs of CPZEN-45, a novel antituberculosis drug.
Ishizaki Y, Takahashi Y, Kimura T, Inoue M, Hayashi C, Igarashi M. Ishizaki Y, et al. J Antibiot (Tokyo). 2019 Dec;72(12):970-980. doi: 10.1038/s41429-019-0225-5. Epub 2019 Aug 30. J Antibiot (Tokyo). 2019. PMID: 31471594 - _N_-Acetylglucosamine-1-Phosphate Transferase, WecA, as a Validated Drug Target in Mycobacterium tuberculosis.
Huszár S, Singh V, Polčicová A, Baráth P, Barrio MB, Lagrange S, Leblanc V, Nacy CA, Mizrahi V, Mikušová K. Huszár S, et al. Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01310-17. doi: 10.1128/AAC.01310-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28874370 Free PMC article. - Mycobacterium tuberculosis Rv1302 and Mycobacterium smegmatis MSMEG_4947 have WecA function and MSMEG_4947 is required for the growth of M. smegmatis.
Jin Y, Xin Y, Zhang W, Ma Y. Jin Y, et al. FEMS Microbiol Lett. 2010 Sep 1;310(1):54-61. doi: 10.1111/j.1574-6968.2010.02045.x. Epub 2010 Jun 23. FEMS Microbiol Lett. 2010. PMID: 20637039 - Caprazamycins: Promising lead structures acting on a novel antibacterial target MraY.
Patel B, Ryan P, Makwana V, Zunk M, Rudrawar S, Grant G. Patel B, et al. Eur J Med Chem. 2019 Jun 1;171:462-474. doi: 10.1016/j.ejmech.2019.01.071. Epub 2019 Mar 20. Eur J Med Chem. 2019. PMID: 30933853 Review. - Caprazamycins: Biosynthesis and structure activity relationship studies.
Wiker F, Hauck N, Grond S, Gust B. Wiker F, et al. Int J Med Microbiol. 2019 Jul;309(5):319-324. doi: 10.1016/j.ijmm.2019.05.004. Epub 2019 May 24. Int J Med Microbiol. 2019. PMID: 31138496 Review.
Cited by
- Novel FR-900493 Analogues That Inhibit the Outgrowth of Clostridium difficile Spores.
Mitachi K, Yun HG, Kurosu SM, Eslamimehr S, Lemieux MR, Klaić L, Clemons WM Jr, Kurosu M. Mitachi K, et al. ACS Omega. 2018 Feb 28;3(2):1726-1739. doi: 10.1021/acsomega.7b01740. Epub 2018 Feb 9. ACS Omega. 2018. PMID: 29503973 Free PMC article. - The mycobacterial cell envelope-lipids.
Jackson M. Jackson M. Cold Spring Harb Perspect Med. 2014 Aug 7;4(10):a021105. doi: 10.1101/cshperspect.a021105. Cold Spring Harb Perspect Med. 2014. PMID: 25104772 Free PMC article. Review. - New liposidomycin congeners produced by Streptomyces sp. TMPU-20A065, anti-Mycobacterium avium complex agents with therapeutic efficacy in a silkworm infection model.
Yagi A, Fujiwara M, Sato M, Abe Y, Uchida R. Yagi A, et al. J Antibiot (Tokyo). 2024 Jul;77(7):412-421. doi: 10.1038/s41429-024-00724-4. Epub 2024 May 8. J Antibiot (Tokyo). 2024. PMID: 38720140 Free PMC article. - The tuberculosis drug discovery and development pipeline and emerging drug targets.
Mdluli K, Kaneko T, Upton A. Mdluli K, et al. Cold Spring Harb Perspect Med. 2015 Jan 29;5(6):a021154. doi: 10.1101/cshperspect.a021154. Cold Spring Harb Perspect Med. 2015. PMID: 25635061 Free PMC article. Review. - Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation.
Beare PA, Jeffrey BM, Long CM, Martens CM, Heinzen RA. Beare PA, et al. PLoS Pathog. 2018 Feb 26;14(3):e1006922. doi: 10.1371/journal.ppat.1006922. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29481553 Free PMC article.
References
- The World Health Organization (2012) Global Tuberculosis Report 2012, www.who.int/tb/publications/global_report/en/
- Centers for Disease Control and Prevention (2006) Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs–worldwide, 2000–2004. MMWR Morb. Mortal Wkly. Rep. 55, 301–305 - PubMed
- Phillips L. (2013) Infectious disease: TB's revenge. Nature 493, 14–16 - PubMed
- Igarashi M., Nakagawa N., Doi N., Hattori S., Naganawa H., Hamada M. (2003) Caprazamycin B, a novel anti-tuberculosis antibiotic, from Streptomyces sp. J. Antibiot. 56, 580–583 - PubMed
- Igarashi M., Takahashi Y., Shitara T., Nakamura H., Naganawa H., Miyake T., Akamatsu Y. (2005) Caprazamycins, novel lipo-nucleoside antibiotics, from Streptomyces sp. II. Structure elucidation of caprazamycins. J. Antibiot. 58, 327–337 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI049151/AI/NIAID NIH HHS/United States
- AI018357/AI/NIAID NIH HHS/United States
- R01 AI018357/AI/NIAID NIH HHS/United States
- AI049151/AI/NIAID NIH HHS/United States
- R56 AI049151/AI/NIAID NIH HHS/United States
- R37 AI018357/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials