Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains - PubMed (original) (raw)
Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains
Christoph H Emmerich et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Polyubiquitin (pUb) chains formed between the C terminus of ubiquitin and lysine 63 (K63) or methionine 1 (M1) of another ubiquitin have been implicated in the activation of the canonical IκB kinase (IKK) complex. Here, we demonstrate that nearly all of the M1-pUb chains formed in response to interleukin-1, or the Toll-Like Receptors 1/2 agonist Pam3CSK4, are covalently attached to K63-pUb chains either directly as K63-pUb/M1-pUb hybrids or indirectly by attachment to the same protein. Interleukin-1 receptor (IL-1R)-associated kinase (IRAK) 1 is modified first by K63-pUb chains to which M1-pUb linkages are added subsequently, and myeloid differentiation primary response gene 88 (MyD88) and IRAK4 are also modified by both K63-pUb and M1-pUb chains. We show that the heme-oxidized IRP2 ubiquitin ligase 1 interacting protein (HOIP) component of the linear ubiquitin assembly complex catalyzes the formation of M1-pUb chains in response to interleukin-1, that the formation of K63-pUb chains is a prerequisite for the formation of M1-pUb chains, and that HOIP interacts with K63-pUb but not M1-pUb linkages. These findings identify K63-Ub oligomers as a major substrate of HOIP in cells where the MyD88-dependent signaling network is activated. The TGF-beta-activated kinase 1 (TAK1)-binding protein (TAB) 2 and TAB3 components of the TAK1 complex and the NFκB Essential Modifier (NEMO) component of the canonical IKK complex bind to K63-pUb chains and M1-pUb chains, respectively. The formation of K63/M1-pUb hybrids may therefore provide an elegant mechanism for colocalizing both complexes to the same pUb chain, facilitating the TAK1-catalyzed activation of IKKα and IKKβ. Our study may help to resolve the debate about the relative importance of K63-pUb and M1-pUb chains in activating the canonical IKK complex.
Keywords: LUBAC; NF-κB; TNF Receptor-Associated Factor 6; Ubc13.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
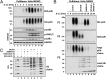
IL-1 induces the formation of K63-pUb/M1-pUb hybrids. (A) IL-1R cells were stimulated for 10 min with 5 ng/mL IL-1β and lysed with 100 mM iodoacetamide to inhibit deubiquitylases, and the pUb chains and associated proteins were captured from the cell extracts with Halo-NEMO. The captured proteins were identified by immunoblotting with antibodies that recognize K63-pUb or M1-pUb chains specifically (Top two subpanels). The extracts (25 µg protein) were immunoblotted with the antibodies indicated (Bottom four subpanels). (B) As in A, except that the pUb chains captured by Halo-NEMO were incubated in the absence (control) or presence of Otulin, AMSH-LP or both deubiquitylases before SDS/PAGE and immunoblotting with antibodies that recognize M1-pUb chains (P1), K63-pUb chains (P2), or with an antibody that recognizes M1-pUb and K63-pUb chains equally well (P3). In P3, the lower half of the membrane was developed for 10 times longer than the upper half. (C) Authentic M1-pUb oligomers (30 ng, lane 1; 10 ng, lane 2) and K63-Ub oligomers (3 ng, lane 7 and 6 ng, lane 8) were used as markers to demonstrate that the small Ub oligomers formed in B after treatment with AMSH-LP were linked via M1 of ubiquitin (lanes 3 and 4) and that those formed by treatment with Otulin (lanes 5 and 6) were linked via K63.
Fig. 2.
Characterization of polyubiquitin chains attached covalently to proteins captured by Halo-NEMO from the extracts of IL-1R cells and THP1 monocytes. (A) IL-1R cells were stimulated with 5 ng/mL IL-1β (Upper) or THP1 monocytes with 1 μg/mL Pam3CSK4 (Lower) and the experiment was then performed as in Fig. 1_B_, except that the proteins captured by Halo-NEMO were treated for 1 h with λPPase (to dephosphorylate IRAK1) in the absence (control) or presence of the deubiquitylases AMSH-LP, Otulin, AMSH-LP plus Otulin, or USP2. The samples were then subjected to SDS/PAGE and immunoblotted for IRAK1. (B) As in A, except that λPPase was omitted and the PVDF membranes were immunoblotted with antibodies that recognize MyD88 and IRAK4. mUb, monoubiquitylated.
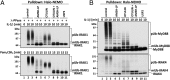
Fig. 3.
Two distinct forms of pUb-IRAK1 identified by the combined use of Halo-NEMO and Halo-TAB2. (A) The experiment was carried out as in Fig. 2_A_ except that pUb chains and associated proteins were captured with Halo-NEMO or Halo-TAB2 [first pull-down (PD)]. Where indicated, the supernatants from the first PD were subjected to a second PD using either Halo-NEMO or Halo-TAB2 as indicated. Captured pUb chains or IRAK1 were identified by immunoblotting. (B) As in A, except that proteins that were captured by Halo-NEMO or Halo-TAB2 from the supernatant of the first Halo-NEMO pull-down, were incubated with Otulin. The ubiquitylation of IRAK1 was then analyzed by immunoblotting with a specific antibody.
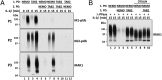
Fig. 4.
Studies on the activity, pUb-binding properties, and role of LUBAC. (A) MEFs from mice expressing either wild-type (wt) HOIP or the HOIP[C879S] mutant were stimulated with 5 ng/mL IL-1α for the times indicated. M1-pUb chains captured from the cell extracts with Halo-NEMO were identified by immunoblotting with a specific antibody (Top). Cell extracts (20 μg protein) were immunoblotted with the antibodies indicated (Bottom seven subpanels). (B) IL-1R cells stably expressing shRNAs specific for HOIP and HOIL-1, or an empty control vector, were stimulated with 5 ng/mL IL-1β and pUb chains captured from extracts with Halo-NEMO or Halo-TAB2. The captured M1-pUb (Top) and K63-pUb chains (Top, second subpanel) were identified by immunoblotting with specific antibodies. Aliquots of the extracts (20 μg protein) were also immunoblotted with the antibodies indicated (Bottom four subpanels). (C) IL-1R cells were not stimulated or stimulated for 15 min with 5 ng/mL IL-1β. The cells were lysed without iodoacetamide to prevent the inactivation of LUBAC, which was then immunoprecipitated from the extracts with anti–HOIL-1, anti-Sharpin, or anti-HOIP. The M1-pUb chains formed after 60 min by LUBAC were examined by immunoblotting. The immunoprecipitates were also immunoblotted for HOIP, HOIL-1, and Sharpin. (D) Full-length GST-HOIP and GST-HOIL-1 were mixed with K63-Ub or M1-Ub oligomers, and, after capture on glutathione-Sepharose, the pUb-oligomers bound to each protein were analyzed by immunoblotting with anti-ubiquitin. Lanes 1 and 6 show, respectively, the K63-pUb oligomers and M1-pUb oligomers used. Lanes 2–5 show the K63-pUb chains and lanes 7–10 the M1-pUb chains captured by each protein.
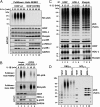
Fig. 5.
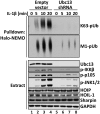
Formation of M1-pUb chains is dependent on the prior formation of K63-pUb chains. IL-1R cells stably expressing shRNA specific for Ubc13 or an empty vector were stimulated for the times indicated with 5 ng/mL IL-1β, and the cells were lysed in the presence of 100 mM iodoacetamide. The pUb chains captured from the cell extracts with Halo-NEMO were subjected to SDS/PAGE and immunoblotting with antibodies that recognize K63-pUb chains (Top) or M1-pUb chains (Top, second subpanel). The cell extracts (20 μg protein) were also subjected to SDS/PAGE and immunoblotted with the antibodies indicated (Bottom eight subpanels).
Fig. 6.
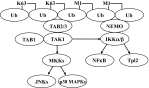
Enhanced activation of the canonical IKK complex via the MyD88-dependent formation of K63/M1-linked hybrid ubiquitin chains. The figure shows schematically how the formation of K63/M1-pUb hybrids may permit the corecruitment of the TAK1 and canonical IKK complexes via the interaction of TAB2/3 with K63-pUb chains and NEMO with M1-pUb chains, thereby facilitating the activation of IKKα and IKKβ by TAK1.
Similar articles
- The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways.
Cohen P, Strickson S. Cohen P, et al. Cell Death Differ. 2017 Jul;24(7):1153-1159. doi: 10.1038/cdd.2017.17. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475177 Free PMC article. Review. - HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation.
Ho YK, Zhi H, Bowlin T, Dorjbal B, Philip S, Zahoor MA, Shih HM, Semmes OJ, Schaefer B, Glover JN, Giam CZ. Ho YK, et al. PLoS Pathog. 2015 Aug 18;11(8):e1005102. doi: 10.1371/journal.ppat.1005102. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26285145 Free PMC article. - Interleukin-1 (IL-1) induces the Lys63-linked polyubiquitination of IL-1 receptor-associated kinase 1 to facilitate NEMO binding and the activation of IkappaBalpha kinase.
Windheim M, Stafford M, Peggie M, Cohen P. Windheim M, et al. Mol Cell Biol. 2008 Mar;28(5):1783-91. doi: 10.1128/MCB.02380-06. Epub 2008 Jan 7. Mol Cell Biol. 2008. PMID: 18180283 Free PMC article. - The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1.
Ordureau A, Smith H, Windheim M, Peggie M, Carrick E, Morrice N, Cohen P. Ordureau A, et al. Biochem J. 2008 Jan 1;409(1):43-52. doi: 10.1042/BJ20071365. Biochem J. 2008. PMID: 17997719 Free PMC article. - Ubiquitin-mediated activation of TAK1 and IKK.
Adhikari A, Xu M, Chen ZJ. Adhikari A, et al. Oncogene. 2007 May 14;26(22):3214-26. doi: 10.1038/sj.onc.1210413. Oncogene. 2007. PMID: 17496917 Review.
Cited by
- Innate immunity kinase TAK1 phosphorylates Rab1 on a hotspot for posttranslational modifications by host and pathogen.
Levin RS, Hertz NT, Burlingame AL, Shokat KM, Mukherjee S. Levin RS, et al. Proc Natl Acad Sci U S A. 2016 Aug 16;113(33):E4776-83. doi: 10.1073/pnas.1608355113. Epub 2016 Aug 1. Proc Natl Acad Sci U S A. 2016. PMID: 27482120 Free PMC article. - E3 ubiquitin ligases Pellinos as regulators of pattern recognition receptor signaling and immune responses.
Medvedev AE, Murphy M, Zhou H, Li X. Medvedev AE, et al. Immunol Rev. 2015 Jul;266(1):109-22. doi: 10.1111/imr.12298. Immunol Rev. 2015. PMID: 26085210 Free PMC article. Review. - The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity.
Damgaard RB, Walker JA, Marco-Casanova P, Morgan NV, Titheradge HL, Elliott PR, McHale D, Maher ER, McKenzie ANJ, Komander D. Damgaard RB, et al. Cell. 2016 Aug 25;166(5):1215-1230.e20. doi: 10.1016/j.cell.2016.07.019. Epub 2016 Aug 11. Cell. 2016. PMID: 27523608 Free PMC article. - M1-linked ubiquitination facilitates NF-κB activation and survival during sterile inflammation.
Aalto A, Martínez-Chacón G, Kietz C, Tsyganova N, Kreutzer J, Kallio P, Broemer M, Meinander A. Aalto A, et al. FEBS J. 2022 Sep;289(17):5180-5197. doi: 10.1111/febs.16425. Epub 2022 Mar 14. FEBS J. 2022. PMID: 35263507 Free PMC article. - Ubiquitin-conjugating enzyme UBE2J1 negatively modulates interferon pathway and promotes RNA virus infection.
Feng T, Deng L, Lu X, Pan W, Wu Q, Dai J. Feng T, et al. Virol J. 2018 Aug 29;15(1):132. doi: 10.1186/s12985-018-1040-5. Virol J. 2018. PMID: 30157886 Free PMC article.
References
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta. 2002;1592(3):265–280. - PubMed
- Ye H, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418(6896):443–447. - PubMed
- Deng L, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103(2):351–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_EX_G0800765/MRC_/Medical Research Council/United Kingdom
- MC_U105192732/MRC_/Medical Research Council/United Kingdom
- 309756/ERC_/European Research Council/International
- MC_EX_UU_G0800765/MRC_/Medical Research Council/United Kingdom
- MC_U127084348/MRC_/Medical Research Council/United Kingdom
- MC_UU_12016/11/MRC_/Medical Research Council/United Kingdom
- G1100713/MRC_/Medical Research Council/United Kingdom
- 100294/Wellcome Trust/United Kingdom
- U105192732/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous