Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies - PubMed (original) (raw)
Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies
Fei Song et al. J Virol. 2013 Nov.
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) has recently emerged as a causative agent of severe respiratory disease in humans. Here, we constructed recombinant modified vaccinia virus Ankara (MVA) expressing full-length MERS-CoV spike (S) protein (MVA-MERS-S). The genetic stability and growth characteristics of MVA-MERS-S make it a suitable candidate vaccine for clinical testing. Vaccinated mice produced high levels of serum antibodies neutralizing MERS-CoV. Thus, MVA-MERS-S may serve for further development of an emergency vaccine against MERS-CoV.
Figures
Fig 1
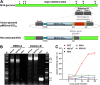
Generating and characterizing recombinant MVA. (A) Schematic diagram of the MVA genome and the locations of major deletion sites I to IV, with deletion III being the site used to insert the MERS-CoV S gene sequences. Flank-1 and flank-2 refer to MVA DNA sequences adjacent to deletion site III which were originally prepared by PCR and cloned into MVA transfer plasmids targeting deletion site III for insertion of recombinant genes. In MVA vector plasmids pIIIH5red-S and -SHA, the S coding gene sequences (MERS-S/SHA) are placed under transcriptional control of the vaccinia virus promoter PmH5 and introduced by homologous recombination between the flanking sequences in the vector and the MVA genome. MVA-MERS-S and MVA-MERS-SHA were isolated in plaque passages by screening for transient coexpression of the fluorescent marker gene mCherry under transcriptional control of the vaccinia virus late promoter P11. Repetitive sequences (del) are designed to remove the mCherry marker by intragenomic homologous recombination (marker gene deletion). (B) Genetic integrity and genetic stability of MVA-MERS-S and MERS-SHA. PCR analysis of genomic viral DNA using oligonucleotide primers to confirm the identity (MERS-S) and proper insertion (deletion III) of S gene sequences. (C) Multiple-step growth analysis of recombinant MVA-MERS-S. Recombinant MVA (MVA-S) and wild-type MVA (MVA) can be efficiently amplified in CEF (multiplicity of infection [MOI], 0.1) but fail to productively grow in HeLa and HaCat human cell lines.
Fig 2
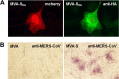
Immunostaining of S proteins in recombinant MVA-infected cells. (A) Transient expression of the marker protein mCherry served to localize single virus-infected cells (left panel). Monoclonal antibody directed against the HA tag (anti-HA) (right panel) reveals the presence of SHA in Vero cells infected (MOI, 0.1) with MVA-MERS-SHA (MVA-SHA). (B) Polyclonal antibodies from a MERS-CoV-infected cynomolgous macaque (anti-MERS-CoV) detected S-producing cell foci in CEF infected with MVA-MERS-S (MVA-S; MOI, 0.1) but no foci when CEF were infected with nonrecombinant MVA (MVA).
Fig 3
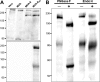
Synthesis of full-length S glycoprotein in recombinant MVA-infected cells. (A) Western blot analysis of cell lysates from MVA-MERS-S (MVA-S)- or MVA-MERS-SHA (MVA-SHA)-infected Vero cells 24 h postinfection. Polypeptides were analyzed by SDS-PAGE and immunoblotting using serum from a MERS-CoV-infected macaque (1:1,000; upper panel) or monoclonal rat anti-HA tag antibody (1:50; lower panel). Lysates from uninfected (Vero) or wild-type MVA-infected (MVA) cells served as controls. (B) Western blot analysis of MVA expressed SHA following treatment with glycosidases. Vero cells were infected with MVA-MERS-SHA for 24 h. Cell lysates were incubated with (+) or without (−) glycosidase PNGase F or endo H and analyzed by SDS-PAGE and immunoblotting with monoclonal rat anti-HA tag antibody (1:50). Numbers on the left indicate molecular masses of marker proteins in kilodaltons.
Fig 4
Antibody responses after intramuscular immunization with recombinant MVA-MERS-S. BALB/c mice (n = 10) were vaccinated twice within a 21-day interval with 108 PFU MVA-MERS-S (MVA-S). Groups of mice (n = 4) vaccinated with wild-type MVA (MVA) or saline (PBS) served as controls. MERS-CoV virus-neutralizing titers (VNT) were determined 20 days after primary immunization (A) and 10 days after the second immunization (B and C) using Huh7 (A and B) and Vero cells (C). Sera obtained after the second boost were also tested against SARS-CoV on Vero cells (D). Serum from a rabbit (17) immunized with the MERS-CoV receptor binding domain (RBD) (E) or sera from mice obtained after the second MVA-S boost (F) were preincubated with PBS, the RBD, or a SARS-CoV-derived control protein (CTRL) at 5 μg/ml for 1 h before incubation with MERS-CoV, followed 1 h later by inoculation on Vero cells.
Similar articles
- Protective Efficacy of Recombinant Modified Vaccinia Virus Ankara Delivering Middle East Respiratory Syndrome Coronavirus Spike Glycoprotein.
Volz A, Kupke A, Song F, Jany S, Fux R, Shams-Eldin H, Schmidt J, Becker C, Eickmann M, Becker S, Sutter G. Volz A, et al. J Virol. 2015 Aug;89(16):8651-6. doi: 10.1128/JVI.00614-15. Epub 2015 May 27. J Virol. 2015. PMID: 26018172 Free PMC article. - Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection.
Jia W, Channappanavar R, Zhang C, Li M, Zhou H, Zhang S, Zhou P, Xu J, Shan S, Shi X, Wang X, Zhao J, Zhou D, Perlman S, Zhang L. Jia W, et al. Emerg Microbes Infect. 2019;8(1):760-772. doi: 10.1080/22221751.2019.1620083. Emerg Microbes Infect. 2019. PMID: 31130102 Free PMC article. - MERS-CoV spike nanoparticles protect mice from MERS-CoV infection.
Coleman CM, Venkataraman T, Liu YV, Glenn GM, Smith GE, Flyer DC, Frieman MB. Coleman CM, et al. Vaccine. 2017 Mar 14;35(12):1586-1589. doi: 10.1016/j.vaccine.2017.02.012. Epub 2017 Feb 23. Vaccine. 2017. PMID: 28237499 Free PMC article. - Antibodies and vaccines against Middle East respiratory syndrome coronavirus.
Xu J, Jia W, Wang P, Zhang S, Shi X, Wang X, Zhang L. Xu J, et al. Emerg Microbes Infect. 2019;8(1):841-856. doi: 10.1080/22221751.2019.1624482. Emerg Microbes Infect. 2019. PMID: 31169078 Free PMC article. Review. - MERS-CoV spike protein: Targets for vaccines and therapeutics.
Wang Q, Wong G, Lu G, Yan J, Gao GF. Wang Q, et al. Antiviral Res. 2016 Sep;133:165-77. doi: 10.1016/j.antiviral.2016.07.015. Epub 2016 Jul 26. Antiviral Res. 2016. PMID: 27468951 Free PMC article. Review.
Cited by
- Implementation of an Immunoassay Based on the MVA-T7pol-Expression System for Rapid Identification of Immunogenic SARS-CoV-2 Antigens: A Proof-of-Concept Study.
Kumar S, Nan L, Kalodimou G, Jany S, Freudenstein A, Brandmüller C, Müller K, Girl P, Ehmann R, Guggemos W, Seilmaier M, Wendtner CM, Volz A, Sutter G, Fux R, Tscherne A. Kumar S, et al. Int J Mol Sci. 2024 Oct 10;25(20):10898. doi: 10.3390/ijms252010898. Int J Mol Sci. 2024. PMID: 39456680 Free PMC article. - Emerging and reemerging infectious diseases: global trends and new strategies for their prevention and control.
Wang S, Li W, Wang Z, Yang W, Li E, Xia X, Yan F, Chiu S. Wang S, et al. Signal Transduct Target Ther. 2024 Sep 11;9(1):223. doi: 10.1038/s41392-024-01917-x. Signal Transduct Target Ther. 2024. PMID: 39256346 Free PMC article. Review. - Dissecting humoral immune responses to an MVA-vectored MERS-CoV vaccine in humans using a systems serology approach.
Weskamm LM, Tarnow P, Harms C, Huchon M, Raadsen MP, Friedrich M, Rübenacker L, Grüttner C, Garcia MG; MVA-MERS-S-CEF study group; Koch T, Becker S, Sutter G, Lhomme E, Haagmans BL, Fathi A, Blois SM, Dahlke C, Richert L, Addo MM. Weskamm LM, et al. iScience. 2024 Jul 8;27(8):110470. doi: 10.1016/j.isci.2024.110470. eCollection 2024 Aug 16. iScience. 2024. PMID: 39148710 Free PMC article. - MVA-based vaccine candidates encoding the native or prefusion-stabilized SARS-CoV-2 spike reveal differential immunogenicity in humans.
Mayer L, Weskamm LM, Fathi A, Kono M, Heidepriem J, Krähling V, Mellinghoff SC, Ly ML, Friedrich M, Hardtke S, Borregaard S, Hesterkamp T, Loeffler FF, Volz A, Sutter G, Becker S, Dahlke C, Addo MM. Mayer L, et al. NPJ Vaccines. 2024 Jan 26;9(1):20. doi: 10.1038/s41541-023-00801-z. NPJ Vaccines. 2024. PMID: 38278816 Free PMC article. - The emergence of SARS-CoV-2 lineages and associated saliva antibody responses among asymptomatic individuals in a large university community.
Merling MR, Williams A, Mahfooz NS, Ruane-Foster M, Smith J, Jahnes J, Ayers LW, Bazan JA, Norris A, Norris Turner A, Oglesbee M, Faith SA, Quam MB, Robinson RT. Merling MR, et al. PLoS Pathog. 2023 Aug 21;19(8):e1011596. doi: 10.1371/journal.ppat.1011596. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37603565 Free PMC article.
References
- Bermingham A, Brown MA, Aarons E, Tong C, Langrish C, Hoschler K, Brown K, Galiano M, Myers R, Pebody RG, Green HK, Boddington NL, Gopal R, Price N, Newsholme W, Drosten C, Fouchier RA, Zambon M. 2012. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to United Kingdom from the Middle East. Euro Surveill. 17:pii=20290 http://www.eurosurveillance.org/viewarticle.aspx?articleid=20290 - PubMed
- van Boheemen S, de Graaf M, Lauber C, Bestebroer TM, Raj VS, Zaki AM, Osterhaus AD, Haagmans BL, Gorbalenya AE, Snijder EJ, Fouchier RA. 2012. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio 3(6):e00473–12.10.1128/mBio.00473-12 - DOI - PMC - PubMed
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RAM. 2012. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367:1814–1820 - PubMed
- Chan JFW, Chan KH, Choi GKY, To KKW, Tse H, Cai JP, Yeung ML, Cheng VC, Chen H, Che XY, Lau SK, Woo PC, Yuen KY. 2013. Differential cell line susceptibility to the emerging novel human betacoronavirus 2c EMC/2012: implications for disease pathogenesis and clinical manifestation. J. Infect. Dis. 207:1743–1752 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources