The other face of restriction: modification-dependent enzymes - PubMed (original) (raw)
Review
The other face of restriction: modification-dependent enzymes
Wil A M Loenen et al. Nucleic Acids Res. 2014 Jan.
Abstract
The 1952 observation of host-induced non-hereditary variation in bacteriophages by Salvador Luria and Mary Human led to the discovery in the 1960s of modifying enzymes that glucosylate hydroxymethylcytosine in T-even phages and of genes encoding corresponding host activities that restrict non-glucosylated phage DNA: rglA and rglB (restricts glucoseless phage). In the 1980's, appreciation of the biological scope of these activities was dramatically expanded with the demonstration that plant and animal DNA was also sensitive to restriction in cloning experiments. The rgl genes were renamed mcrA and mcrBC (modified cytosine restriction). The new class of modification-dependent restriction enzymes was named Type IV, as distinct from the familiar modification-blocked Types I-III. A third Escherichia coli enzyme, mrr (modified DNA rejection and restriction) recognizes both methylcytosine and methyladenine. In recent years, the universe of modification-dependent enzymes has expanded greatly. Technical advances allow use of Type IV enzymes to study epigenetic mechanisms in mammals and plants. Type IV enzymes recognize modified DNA with low sequence selectivity and have emerged many times independently during evolution. Here, we review biochemical and structural data on these proteins, the resurgent interest in Type IV enzymes as tools for epigenetic research and the evolutionary pressures on these systems.
Figures
Figure 1.
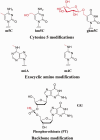
DNA modifications recognized by Type IV enzymes. Enzymatic DNA modifications in the major groove of double-stranded DNA are methylation at cytosine C5 or N4, or at adenosine N6; and glucosylation of a pre-existing 5-hydroxymethylcytosine. The beta-glucosyl derivative is shown; other configurations and other sugars are known to be added by some phages. hm5C is incorporated during replication, after conversion of the dCTP pool to hmdCTP. Phosphorothioate modification of the backbone is carried out postsynthetically. Other biological DNA modifications are known. Only those shown to elicit action of characterized Type IV enzymes are shown here.
Figure 2.
McrA functional domains. Domain function was inferred indirectly from genetic analysis by Anton & Raleigh (2004) (57). Many mutations in the N-terminal domain spared some activity in one or more of three functional tests (grey segments) while others were deficient in all activities (black segments). One mutation (asterix) was fully active on m5C-containing substrates, but fully inactive in the hm5C challenge in vivo. Most mutations in the C-terminus (pale grey segment) retained function in one test that was interpreted as measuring m5C binding ability. A predicted structural model by Bujnicki, Radlinska and Rychlewski (2000) (58) for this C-terminal region is compatible with these results.
Figure 3.
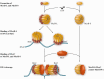
McrBC Assembly Model. Two proteins are expressed from mcrB in vivo. Both the complete protein (McrB-L) and a small one missing the N-terminus (McrB-S; top row) bind GTP, forming high-order multimers detected by gel filtration (second row). When visualized by scanning transmission electron microscopy, these appear as heptameric rings with a central channel. Rings of McrB-L in top views show projections that may correspond to the N-terminal DNA-binding domain (red segment). Both forms can then associate with McrC, judged again by gel filtration. McrB-L:GTP can bind to its specific substrate (RmC) in the absence of McrC (third row); in its presence, the substrate is cleaved (fourth row). GTP hydrolysis is required for cleavage (arrow): a supershifted binding complex forms in the presence of GTP-gamma-S, but no cleavage occurs. Translocation accompanies GTP hydrolysis; double-stranded cleavage requires collaboration between two complexes, or a translocation block. The path of the DNA in the figure is arbitrary, as is the conformation of McrC. Modified from Bourniquel,A.A. and Bickle,T.A. Complex restriction enzymes: NTP-driven molecular motors. Bourniquel and Bickle (84) with permission. Copyright © 2002 Elsevier Masson SAS. All rights reserved.
Figure 4.
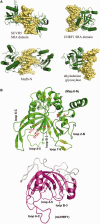
McrB-N in comparison to other base-flipping proteins. (A) SRA domains SUVH5 (3Q0C) and UHRF1 (2ZKD) use loops extending from a crescent formed from two beta sheets to flip C or m5C from undeformed B-form DNA into a pocket (top row), whereas McrB-N (3SSC; bottom row) uses loops from one beta-sheet to distort the DNA and flip the base. It resembles the human alkyladenine glycosylase (1BNK) (bottom row) in bending the DNA toward the major groove, while flipping the base via the minor groove. Figure 5 of Sukackaite et al. (60). (B) The SRA-like hemi-methylated 5mC recognition domains. A ribbon model of the N-terminal domain of the MspJI structure (4F0Q and 4F0P; left) compared with the SRA domain of URHF1 (PDB 3FDE; right). The crescent shape formed by interacting beta sheets and helices αB and αC are the conserved features of the SRA domain highlighted here. Loops on the concave side of UHRF1 participate in flipping the base, and similar loops presumably do so for MspJI. Two of these vary in length among family members and may play roles in sequence context specificity. Figure 2a and b from Horton et al. (80).
Figure 5.
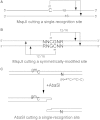
Schematic diagrams of cleavage positions for MspJI and AbaSDFI. Cleavage of both strands is elicited by a singly modified site for both MspJI (A) and AbaSDFI (C). Cleavage position is fixed relative to the modified site, but with a four-base 5′ extension for MspJI and a two-base 3′ extension for AbaSDFI. When a site is symmetrically-modified (as for CpG sites in mammalian DNA), a 32 base-pair fragment is excised from the DNA (B). (A) Figure 2a and (B) Figure 3a reprinted with permission from Cohen-Karni et al. (52). (C) Figure 5a from Wang et al. (47).
Similar articles
- Genetic and physical mapping of the mcrA (rglA) and mcrB (rglB) loci of Escherichia coli K-12.
Raleigh EA, Trimarchi R, Revel H. Raleigh EA, et al. Genetics. 1989 Jun;122(2):279-96. doi: 10.1093/genetics/122.2.279. Genetics. 1989. PMID: 2548920 Free PMC article. - A unique family of Mrr-like modification-dependent restriction endonucleases.
Zheng Y, Cohen-Karni D, Xu D, Chin HG, Wilson G, Pradhan S, Roberts RJ. Zheng Y, et al. Nucleic Acids Res. 2010 Sep;38(16):5527-34. doi: 10.1093/nar/gkq327. Epub 2010 May 5. Nucleic Acids Res. 2010. PMID: 20444879 Free PMC article. - Structural and functional diversity among Type III restriction-modification systems that confer host DNA protection via methylation of the N4 atom of cytosine.
Murray IA, Luyten YA, Fomenkov A, Dai N, Corrêa IR Jr, Farmerie WG, Clark TA, Korlach J, Morgan RD, Roberts RJ. Murray IA, et al. PLoS One. 2021 Jul 6;16(7):e0253267. doi: 10.1371/journal.pone.0253267. eCollection 2021. PLoS One. 2021. PMID: 34228724 Free PMC article. - ATP-dependent restriction enzymes.
Rao DN, Saha S, Krishnamurthy V. Rao DN, et al. Prog Nucleic Acid Res Mol Biol. 2000;64:1-63. doi: 10.1016/s0079-6603(00)64001-1. Prog Nucleic Acid Res Mol Biol. 2000. PMID: 10697406 Review. - Complex restriction enzymes: NTP-driven molecular motors.
Bourniquel AA, Bickle TA. Bourniquel AA, et al. Biochimie. 2002 Nov;84(11):1047-59. doi: 10.1016/s0300-9084(02)00020-2. Biochimie. 2002. PMID: 12595133 Review.
Cited by
- EcoBLMcrX, a classical modification-dependent restriction enzyme in Escherichia coli B: Characterization in vivo and in vitro with a new approach to cleavage site determination.
Fomenkov A, Sun Z, Dila DK, Anton BP, Roberts RJ, Raleigh EA. Fomenkov A, et al. PLoS One. 2017 Jun 27;12(6):e0179853. doi: 10.1371/journal.pone.0179853. eCollection 2017. PLoS One. 2017. PMID: 28654677 Free PMC article. - Methylation Warfare: Interaction of Pneumococcal Bacteriophages with Their Host.
Furi L, Crawford LA, Rangel-Pineros G, Manso AS, De Ste Croix M, Haigh RD, Kwun MJ, Engelsen Fjelland K, Gilfillan GD, Bentley SD, Croucher NJ, Clokie MR, Oggioni MR. Furi L, et al. J Bacteriol. 2019 Sep 6;201(19):e00370-19. doi: 10.1128/JB.00370-19. Print 2019 Oct 1. J Bacteriol. 2019. PMID: 31285240 Free PMC article. - Phage based vaccine: A novel strategy in prevention and treatment.
Mohammad Hasani S, Ghafouri E, Kouhpayeh S, Amerizadeh F, Rahimmanesh I, Amirkhani Z, Khanahmad H. Mohammad Hasani S, et al. Heliyon. 2023 Sep 15;9(9):e19925. doi: 10.1016/j.heliyon.2023.e19925. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809683 Free PMC article. Review. - Engineering nicking enzymes that preferentially nick 5-methylcytosine-modified DNA.
Gutjahr A, Xu SY. Gutjahr A, et al. Nucleic Acids Res. 2014 May;42(9):e77. doi: 10.1093/nar/gku192. Epub 2014 Mar 7. Nucleic Acids Res. 2014. PMID: 24609382 Free PMC article. - Type II restriction endonucleases--a historical perspective and more.
Pingoud A, Wilson GG, Wende W. Pingoud A, et al. Nucleic Acids Res. 2014 Jul;42(12):7489-527. doi: 10.1093/nar/gku447. Epub 2014 May 30. Nucleic Acids Res. 2014. PMID: 24878924 Free PMC article. Review.
References
- Revel HR, Luria SE. DNA-glucosylation in T-even phage: genetic determination and role in phage-host interaction. Annu. Rev. Genet. 1970;4:177–192. - PubMed
- Noyer-Weidner M, Diaz R, Reiners L. Cytosine-specific DNA modification interferes with plasmid establishment in Escherichia coli K12: involvement of rglB. Mol. Gen. Genet. 1986;205:469–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases