Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression - PubMed (original) (raw)
. 2013 Sep 12;4(5):913-20.
doi: 10.1016/j.celrep.2013.07.030. Epub 2013 Aug 29.
Jie Liu, Edmund B Chen, Jennifer J Wang, Liu Cao, Nisha Narayan, Marie M Fergusson, Ilsa I Rovira, Michele Allen, Danielle A Springer, Cory U Lago, Shuling Zhang, Wendy DuBois, Theresa Ward, Rafael deCabo, Oksana Gavrilova, Beverly Mock, Toren Finkel
Affiliations
- PMID: 23994476
- PMCID: PMC3784301
- DOI: 10.1016/j.celrep.2013.07.030
Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression
J Julie Wu et al. Cell Rep. 2013.
Abstract
We analyzed aging parameters using a mechanistic target of rapamycin (mTOR) hypomorphic mouse model. Mice with two hypomorphic (mTOR(Δ/Δ)) alleles are viable but express mTOR at approximately 25% of wild-type levels. These animals demonstrate reduced mTORC1 and mTORC2 activity and exhibit an approximately 20% increase in median survival. While mTOR(Δ/Δ) mice are smaller than wild-type mice, these animals do not demonstrate any alterations in normalized food intake, glucose homeostasis, or metabolic rate. Consistent with their increased lifespan, mTOR(Δ/Δ) mice exhibited a reduction in a number of aging tissue biomarkers. Functional assessment suggested that, as mTOR(Δ/Δ) mice age, they exhibit a marked functional preservation in many, but not all, organ systems. Thus, in a mammalian model, while reducing mTOR expression markedly increases overall lifespan, it affects the age-dependent decline in tissue and organ function in a segmental fashion.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
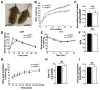
A mouse model of reduced mTOR expression extends lifespan. A) Genomic organization of the wild type allele (+) and the hypomorphic mTOR allele (Δ). B) Representative mTOR protein expression in the liver of two wild type (mTOR+/+) and two mTORΔ/Δ mice. GAPDH is used as a loading control and the normalized expression (WT=1) of mTOR to GAPDH is shown for each animal. C) Leucine-stimulated S6 Kinase phosphorylation (pS6K) in primary mouse embryonic fibroblasts isolated from wild type or mTORΔ/Δ mice. D) Insulin-stimulated mTOR activity in pairs of wild type or mTORΔ/Δ mice. E) Survival of a cohort of male WT and mTORΔ/Δ mice. F) Survival of female members of the cohort. G) Survival of the overall cohort. H) Incidence of malignant tumors found at necropsy denoted by shaded portion of each bar. While the overall incidence of cancer was different between the two genotypes, the spectrum of tumors observed was similar. **p<0.001 Fisher’s Exact test.
Figure 2
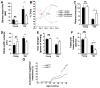
The mTORΔ/Δ mice are smaller but have no significant alterations in glucose homeostasis and metabolism. All measurements were performed using male mice. A) Representative size of a wild type and mTORΔ/Δ adult mouse. B) Body weight of wild type and mTORΔ/Δ mice (n=7 WT and n=7 mTORΔ/Δ, curves are statistically different using a one-way Anova followed by two tailed t-test, p<0.01). C) Daily food intake is indistinguishable between wild type (shaded bar) and mTORΔ/Δ mice (open bar) (n=7 WT and n=6 mTORΔ/Δ, food intake is normalized to body weight). D) Glucose tolerance of 8–12 week old wild type and mTORΔ/Δ mice (n=7 WT and n=6 mTORΔ/Δ). E) Insulin tolerance test of 8–12 week old wild type and mTORΔ/Δ mice (n=12 WT and n=7 mTORΔ/Δ). F) Respiratory exchange ratio (RER) of WT (n=7) and mTORΔ/Δ (n=5). G) Measurement of rates of total body fatty acid oxidation normalized to body weight in WT (n=7) and mTORΔ/Δ mice (n=5). H) Total oxygen consumption normalized to body weight (n=7 WT and n=5 mTORΔ/Δ). I) Total daily energy expenditure is not altered in mTORΔ/Δ mice (n=7 WT and n=5 mTORΔ/Δ). For all panels, shaded bars represent the wild type mice and the open bars represent the mTORΔ/Δ mice. Where indicated, metabolic parameters are adjusted to body weight raised to the 0.75 power as indicated by the symbol (BW). All pooled data is presented as mean ± SEM.
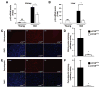
Figure 3
Molecular and biochemical biomarkers of aging are reduced in old mTORΔ/Δ mice. A) Assessment of the age-dependent increase in kidney mRNA levels for the cell cycle inhibitor p16INK4a normalized to GAPDH expression (n=3 mice per genotype and age with each animal performed in triplicate). B) A similar assessment in old and young liver samples (n=6 young WT, n=5 young mTORΔ/Δ, n=4 old WT and n=4 old mTORΔ/Δ with each sample performed in triplicate). C) Representative brain sections stained for nitrotyrosine (red, upper panels) obtained from young WT mice, old WT mice and old mTORΔ/Δ mice. Cell nuclei with stained concurrently with DAPI (blue, lower panels). D) Intensity of nitrotyrosine staining in the brains of old WT (n=3 animals with three to five determinations per animal) and mTORΔ/Δ (n=4 animals with three to five determinations per animal) mice. E) Staining for polyubquitinated proteins in brain tissue sections obtained from young WT mice, old WT mice or old mTORΔ/Δ mice. Upper panels (red) are stained with an antibody that recognizes proteins that are polyubiquitinated, lower panels are analyzed by nuclear DAPI staining. F) Quantification of polyubiquitinated protein levels in brain sections of WT (n=3 animals with three to five determinations per animal) and mTORΔ/Δ (n=4 animals with three to five determinations per animal) mice. All pooled data is presented as mean ± SEM. *p<0.05, **p<0.01.
Figure 4
The effects of reduced mTOR expression on a range of tissue specific age-related parameters. A) Escape latency times on day 3 of training for the Barnes maze test for both young female (n=6 mice per genotype) and old female (n=9 WT and n=13 mTORΔ/Δ) mice. Wild type mice are shown in the shaded bars, while the open bars are mTORΔ/Δ mice. *p <0.05. B) Learning strategy of old mice in the acquisition phase for training in the Barnes maze. Arrows indicate transition point between random to directed searching, an indicator of the speed in which new spatial learning is obtained (n=9 WT female mice and n=13 mTORΔ/Δ female mice). C) Duration on the Rotarod, a measure of coordination and balance (n=6 male mice per genotype for young animals and n=4 old male WT, n=7 old male mTORΔ/Δ mice, *p <0.05). D) Stride width variance in young and old mice (n= 6 young female mice per genotype and n=6 old WT male and female mice and n=13 old male and female mTORΔ/Δ mice, *p <0.05). E) Grip strength, normalized to gram of body weight, in young female mice (n=6 per genotype) and old female mice (n=4 WT and n=11 for mTORΔ/Δ mice, *p <0.05). F) Assessment of the age-dependent decline in bone volume (BV) to tissue volume (TV) (n= 4 young mice per genotype and n=6 old mice per genotype, *p <0.05). G) Age-dependent incidence of visibly apparent superficial infections of the skin, eyes or mouth of the total cohort of wild type and mTORΔ/Δ mice (n=34 WT and n=43 mTORΔ/Δ mice, statistical analysis by Fisher’s Exact test, *p<0.05, **p<0.01). All bar graph data is presented as mean ± SEM.
Similar articles
- Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan.
Lamming DW, Mihaylova MM, Katajisto P, Baar EL, Yilmaz OH, Hutchins A, Gultekin Y, Gaither R, Sabatini DM. Lamming DW, et al. Aging Cell. 2014 Oct;13(5):911-7. doi: 10.1111/acel.12256. Epub 2014 Jul 25. Aging Cell. 2014. PMID: 25059582 Free PMC article. - mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review.
Weichhart T. Weichhart T. Gerontology. 2018;64(2):127-134. doi: 10.1159/000484629. Epub 2017 Dec 1. Gerontology. 2018. PMID: 29190625 Free PMC article. Review. - Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging.
Arriola Apelo SI, Lin A, Brinkman JA, Meyer E, Morrison M, Tomasiewicz JL, Pumper CP, Baar EL, Richardson NE, Alotaibi M, Lamming DW. Arriola Apelo SI, et al. Elife. 2020 Jul 28;9:e56177. doi: 10.7554/eLife.56177. Elife. 2020. PMID: 32720643 Free PMC article. - Comparative idiosyncrasies in life extension by reduced mTOR signalling and its distinctiveness from dietary restriction.
Garratt M, Nakagawa S, Simons MJ. Garratt M, et al. Aging Cell. 2016 Aug;15(4):737-43. doi: 10.1111/acel.12489. Epub 2016 May 3. Aging Cell. 2016. PMID: 27139919 Free PMC article. - Dissecting mammalian target of rapamycin to promote longevity.
Mendelsohn AR, Larrick JW. Mendelsohn AR, et al. Rejuvenation Res. 2012 Jun;15(3):334-7. doi: 10.1089/rej.2012.1347. Rejuvenation Res. 2012. PMID: 22758368 Review.
Cited by
- IGF2BP2/IMP2-Deficient mice resist obesity through enhanced translation of Ucp1 mRNA and Other mRNAs encoding mitochondrial proteins.
Dai N, Zhao L, Wrighting D, Krämer D, Majithia A, Wang Y, Cracan V, Borges-Rivera D, Mootha VK, Nahrendorf M, Thorburn DR, Minichiello L, Altshuler D, Avruch J. Dai N, et al. Cell Metab. 2015 Apr 7;21(4):609-21. doi: 10.1016/j.cmet.2015.03.006. Cell Metab. 2015. PMID: 25863250 Free PMC article. - A metabolic perspective of Peto's paradox and cancer.
Dang CV. Dang CV. Philos Trans R Soc Lond B Biol Sci. 2015 Jul 19;370(1673):20140223. doi: 10.1098/rstb.2014.0223. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26056367 Free PMC article. Review. - 10-Hydroxy-2-decenoic Acid, the Major Lipid Component of Royal Jelly, Extends the Lifespan of Caenorhabditis elegans through Dietary Restriction and Target of Rapamycin Signaling.
Honda Y, Araki Y, Hata T, Ichihara K, Ito M, Tanaka M, Honda S. Honda Y, et al. J Aging Res. 2015;2015:425261. doi: 10.1155/2015/425261. Epub 2015 Feb 19. J Aging Res. 2015. PMID: 25789174 Free PMC article. - An overview of rapamycin: from discovery to future perspectives.
Yoo YJ, Kim H, Park SR, Yoon YJ. Yoo YJ, et al. J Ind Microbiol Biotechnol. 2017 May;44(4-5):537-553. doi: 10.1007/s10295-016-1834-7. Epub 2016 Sep 9. J Ind Microbiol Biotechnol. 2017. PMID: 27613310 Review. - Lifespan extension and cancer prevention in HER-2/neu transgenic mice treated with low intermittent doses of rapamycin.
Popovich IG, Anisimov VN, Zabezhinski MA, Semenchenko AV, Tyndyk ML, Yurova MN, Blagosklonny MV. Popovich IG, et al. Cancer Biol Ther. 2014 May;15(5):586-92. doi: 10.4161/cbt.28164. Epub 2014 Feb 20. Cancer Biol Ther. 2014. PMID: 24556924 Free PMC article.
References
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. - PubMed
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous