Phosphate concentration and the putative sensor kinase protein CckA modulate cell lysis and release of the Rhodobacter capsulatus gene transfer agent - PubMed (original) (raw)
Phosphate concentration and the putative sensor kinase protein CckA modulate cell lysis and release of the Rhodobacter capsulatus gene transfer agent
A B Westbye et al. J Bacteriol. 2013 Nov.
Abstract
The gene transfer agent of Rhodobacter capsulatus (RcGTA) is a bacteriophage-like genetic element with the sole known function of horizontal gene transfer. Homologues of RcGTA genes are present in many members of the alphaproteobacteria and may serve an important role in microbial evolution. Transcription of RcGTA genes is induced as cultures enter the stationary phase; however, little is known about cis-active sequences. In this work, we identify the promoter of the first gene in the RcGTA structural gene cluster. Additionally, gene transduction frequency depends on the growth medium, and the reason for this is not known. We report that millimolar concentrations of phosphate posttranslationally inhibit the lysis-dependent release of RcGTA from cells in both a complex medium and a defined medium. Furthermore, we found that cell lysis requires the genes rcc00555 and rcc00556, which were expressed and studied in Escherichia coli to determine their predicted functions as an endolysin and holin, respectively. Production of RcGTA is regulated by host systems, including a putative histidine kinase, CckA, and we found that CckA is required for maximal expression of rcc00555 and for maturation of RcGTA to yield gene transduction-functional particles.
Figures
Fig 1
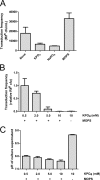
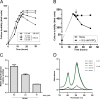
High concentrations of phosphate inhibit gene transduction. The transduction frequencies of SB1003 culture supernatants are shown. (A) Transduction frequencies of cultures grown in YPSm medium (None) or YPSm supplemented with 10 mM KPO4, NaPO4, or MOPS; (B) transduction frequencies of cultures grown in RCVm medium containing various concentrations of KPO4 (0.5, 2.0, 5.0, and 10 mM); (C) pH of culture supernatant at harvest. Error bars represent the standard deviations of three biological replicates. Samples were normalized to the culture turbidity at 660 nm (A and B).
Fig 2
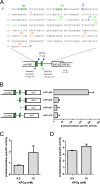
Mapping of the RcGTA promoter and effect of phosphate concentration on RcGTA gene transcription. (A) Annotated sequence and schematic representation of the promoter region of ORF g1 of the RcGTA promoter region. Dark green and light green, −10 and −35 boxes manually annotated and identified by BPROM, respectively; blue, 5′ ends of mRNA identified using 5′ RACE; orange, putative translational start codons for ORF g1 from the literature; red asterisk, annotated stop codon of rcc01681. (B) Schematic representation of ORF g1::lacZ fusions constructs (left) and β-galactosidase activities of strain Y262 containing the indicated plasmid (right); plasmid names ending in -g61, -g64, and -g65 encode fusions to putative start codon 5, whereas plasmid p601-g15 encodes a fusion to putative start codon 4. (C) β-Galactosidase specific activities of WT strain SB1003 containing ORF g1::lacZ fusion plasmid p601-g65 grown in RCVm containing 0.5 mM or 10 mM KPO4 for 36 h. (D) β-Galactosidase specific activities of strain SB555 containing the ORF g1::lacZ fusion plasmid p601-g65 grown in RCVm containing 0.5 mM or 10 mM KPO4 for 24 h. Error bars represent the standard deviations of three biological replicates.
Fig 3
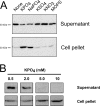
Release of RcGTA is inhibited by high phosphate concentrations. Western blots of culture supernatant and cell pellet fractions probed using RcGTA capsid protein antiserum are shown. (A) Western blots of cells grown in YPSm complex medium (None) and YPSm supplemented with 10 mM KPO4, NaPO4, KSO4, KNO3, or MOPS, as indicated; (B) Western blots of cells grown in RCVm defined medium containing the indicated concentrations of KPO4. Samples were normalized to the culture turbidity at 660 nm.
Fig 4
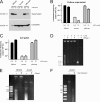
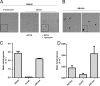
Independent roles of the phosphate concentration and the CckA protein in production and release of mature RcGTA particles. (A) Western blots of strains DE442, DE442 Δ_cckA_, and DE442 Δ_cckA_ complemented in trans by pRCckA. Culture supernatant and cell pellet fractions were probed using RcGTA capsid protein antiserum. The approximate migration of the 30-kDa marker is indicated. (B) Transduction frequencies of culture supernatants of DE442, DE442 Δ_cckA_, and DE442 Δ_cckA_ complemented in trans by pRCckA. (C) Transduction frequencies of freeze-thaw-lysed cells of DE442, DE442 Δ_cckA_, and DE442 Δ_cckA_ complemented in trans by pRCckA. (D) Native agarose gel of RcGTA. Lanes 1 and 2, filtered DE442 culture supernatant; lanes 3 and 4, resuspended pellet of supernatant after ultracentrifugation; lanes 5 and 6, heat-treated samples. (E) Native agarose gel comparing DE442 and DE442 Δ_cckA_ lysates obtained by passage through a French press; cells were cultured in RCV. (F) Native agarose gel of DE442 Δ_cckA_ lysate obtained by passage through a French press subjected to DNase I, followed by heat treatment. Cells were cultured in RCVm medium (A, B, C, E) or YPS (D). Samples were normalized to the total amount of protein in French press lysates of culture (A and B) or cell pellet (C). Error bars represent the standard deviations of three biological replicates.
Fig 5
A decrease in culture turbidity and the release of a cytoplasmic enzyme and intracellular pigments indicate modulation of cell lysis by phosphate. (A) Kinetics of changes in culture turbidity of the overproducer DE442 and WT SB1003 strains grown in the presence of several concentrations of phosphate; (B) kinetics of change in the culture turbidity of overproducer DE442 after addition of phosphate; (C) extracellular fraction of cytoplasmic enzyme malate dehydrogenase relative to the total (extracellular plus intracellular) specific activity in DE442 cultures grown in the presence of different concentrations of phosphate; (D) absorption spectra of cell-free culture supernatants of strains DE442, DE442 Δ_cckA_, DE442 Δ_cckA_ complemented in trans with pRCckA, and DE555 in the region from 750 to 950 nm, showing transmembrane LH2 complex peaks at 802 and 855 nm. Samples were blanked against sterile RCVm medium. All samples were from cultures grown in RCVm containing 0.5 mM KPO4, unless otherwise indicated. Growth curves (A and B) show the average of two biological samples. Error bars represent the standard deviations of three biological replicates.
Fig 6
Production of spheroplast-like vesicles accompanies RcGTA release, and CckA is required for maximal cell lysis. (A) Phase-contrast microscopy of pellet layers after centrifugation of DE442 cells; an upper translucent layer and a lower dense layer, with and without lysozyme treatment, are indicated; (B) phase-contrast microscopy of a resuspended pellet of an ultracentrifuged supernatant from an SB1003 culture shows the presence of spheroplast-like structures (arrow). (Insets) Digital enlargements. Magnifications, ×100. (C) Malate dehydrogenase specific activities of culture supernatants of DE442, DE442 Δ_cckA_, and DE442 Δ_cckA_ complemented in trans with pRCckA. (D) Activity of malate dehydrogenase in culture supernatants of SB1003, SB1003 Δ_cckA_, and SB1003 Δ_cckA_ complemented in trans with pRCckA. All samples were from cultures grown in RCVm containing 0.5 mM KPO4, unless otherwise indicated. Error bars represent the standard deviations of three biological replicates.
Fig 7
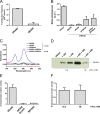
The rcc00555 endolysin is essential for maximal release of RcGTA by cell lysis and requires CckA for expression that is independent of the phosphate concentration. Comparisons of parental strain DE442, mutant DE555, and DE555 complemented in trans with plasmids pR555 and pI556Gm, either singly or together, are shown (A to D). (A) Transduction frequencies of cells cultured in RCVm containing 0.5 mM KPO4. (B) Extracellular levels of cytoplasmic enzyme malate dehydrogenase activity of cells cultured in RCVm containing 0.5 mM KPO4. (C) Absorption spectra of cell-free culture supernatants in the region from 750 nm to 950 nm, showing transmembrane LH2 complex peaks at 802 and 855 nm. Samples were blanked against sterile RCVm medium. (D) Western blots of culture supernatant fractions of DE555 and DE555 complemented with plasmid pR555 and/or pI556Gm probed using RcGTA capsid protein antiserum. The KPO4 concentration of the medium and the approximate migration of the 30-kDa marker are indicated. (E) Promoter activity of the rcc00555 endolysin measured by determination of the β-galactosidase activity in DE442, DE442 Δ_cckA_, and SB1003 strains containing reporter plasmid pXCA-555 cultured in RCV medium (9.6 mM KPO4). (F) rcc00555 promoter activity measured by determination of the β-galactosidase activity of DE555(pXCA-555) cultured in RCVm defined medium containing 0.5 mM or 10 mM KPO4. Error bars represent the standard deviations of a minimum of three biological replicates (A, B, F) or the ranges for two biological replicates (E).
Fig 8
Expression of the rcc00555 endolysin and rcc00556 holin in E. coli. The lytic and peptidoglycan-degrading activities of rcc00555 (A and B) and holin-like activity of rcc00556 (C and D) expressed in E. coli are shown. (A) Turbidity after resuspension in dH2O of induced E. coli. (B) Lane 1, Zymogram of E. coli culture lysate; lane 2, affinity-purified 555C protein; lane 3, SDS-PAGE of purified 555C protein. The approximate migration of the markers is indicated. (C) Growth curves of E. coli. Arrow, induction time point. (D) Propidium iodide staining of E. coli after induction of rcc00556 expression. Cells contained the inducible rcc00555 expression plasmids pET-555C (C-terminally 6-His tagged) or the empty vector control pET28a(+) (A and B) and the inducible rcc00556 expression plasmid pI556 or the empty vector control pIND4 (C and D). Cells were induced by addition of 1 mM IPTG. Error bars represent the ranges for two (A) or the standard deviations for three (C) biological replicates.
Fig 9
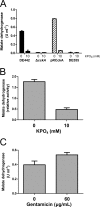
Inhibition of cell lysis by phosphate occurs in the absence of de novo protein synthesis. The malate dehydrogenase activity in cell-free supernatants is shown. (A) DE442, DE442 Δ_cckA_, DE442 Δ_cckA_(pRCckA), and DE555 cells resuspended in RCVm defined medium lacking phosphate (bars labeled 0) or containing 10 mM added phosphate (bars labeled 10); (B) DE442 resuspended in Tris-HCl buffer containing no (bars labeled 0) or 10 mM (bars labeled 10) added phosphate (C) DE442 treated with 0 or 60 μg/ml gentamicin and resuspended in RCVm defined medium lacking phosphate. Cells were resuspended to an OD660 of 5.0 (A and B) or 0.5 (C). For panel B, activity is expressed relative to the activity in RCVm lacking phosphate. For panel C, activity was multiplied by 10 for direct comparison with experiments using an OD660 of 5.0. Error bars represent the standard deviations of three biological replicates.
Similar articles
- The SOS Response Master Regulator LexA Regulates the Gene Transfer Agent of Rhodobacter capsulatus and Represses Transcription of the Signal Transduction Protein CckA.
Kuchinski KS, Brimacombe CA, Westbye AB, Ding H, Beatty JT. Kuchinski KS, et al. J Bacteriol. 2016 Feb 1;198(7):1137-48. doi: 10.1128/JB.00839-15. J Bacteriol. 2016. PMID: 26833411 Free PMC article. - The Protease ClpXP and the PAS Domain Protein DivL Regulate CtrA and Gene Transfer Agent Production in Rhodobacter capsulatus.
Westbye AB, Kater L, Wiesmann C, Ding H, Yip CK, Beatty JT. Westbye AB, et al. Appl Environ Microbiol. 2018 May 17;84(11):e00275-18. doi: 10.1128/AEM.00275-18. Print 2018 Jun 1. Appl Environ Microbiol. 2018. PMID: 29625982 Free PMC article. - The Gene Transfer Agent RcGTA Contains Head Spikes Needed for Binding to the Rhodobacter capsulatus Polysaccharide Cell Capsule.
Westbye AB, Kuchinski K, Yip CK, Beatty JT. Westbye AB, et al. J Mol Biol. 2016 Jan 29;428(2 Pt B):477-91. doi: 10.1016/j.jmb.2015.12.010. Epub 2015 Dec 19. J Mol Biol. 2016. PMID: 26711507 - The gene transfer agent of Rhodobacter capsulatus.
Leung MM, Florizone SM, Taylor TA, Lang AS, Beatty JT. Leung MM, et al. Adv Exp Med Biol. 2010;675:253-64. doi: 10.1007/978-1-4419-1528-3_14. Adv Exp Med Biol. 2010. PMID: 20532745 Review. - Importance of widespread gene transfer agent genes in alpha-proteobacteria.
Lang AS, Beatty JT. Lang AS, et al. Trends Microbiol. 2007 Feb;15(2):54-62. doi: 10.1016/j.tim.2006.12.001. Epub 2006 Dec 20. Trends Microbiol. 2007. PMID: 17184993 Review.
Cited by
- Gene Transfer Agents in Bacterial Endosymbionts of Microbial Eukaryotes.
George EE, Tashyreva D, Kwong WK, Okamoto N, Horák A, Husnik F, Lukeš J, Keeling PJ. George EE, et al. Genome Biol Evol. 2022 Jul 2;14(7):evac099. doi: 10.1093/gbe/evac099. Genome Biol Evol. 2022. PMID: 35738252 Free PMC article. - Identification and characterization of a direct activator of a gene transfer agent.
Fogg PCM. Fogg PCM. Nat Commun. 2019 Feb 5;10(1):595. doi: 10.1038/s41467-019-08526-1. Nat Commun. 2019. PMID: 30723210 Free PMC article. - Control of a gene transfer agent cluster in Caulobacter crescentus by transcriptional activation and anti-termination.
Tran NT, Le TBK. Tran NT, et al. Nat Commun. 2024 Jun 4;15(1):4749. doi: 10.1038/s41467-024-49114-2. Nat Commun. 2024. PMID: 38834569 Free PMC article. - Gene Transfer Agents in Symbiotic Microbes.
Christensen S, Serbus LR. Christensen S, et al. Results Probl Cell Differ. 2020;69:25-76. doi: 10.1007/978-3-030-51849-3_2. Results Probl Cell Differ. 2020. PMID: 33263868 Review. - Insights into origin and evolution of α-proteobacterial gene transfer agents.
Shakya M, Soucy SM, Zhaxybayeva O. Shakya M, et al. Virus Evol. 2017 Dec 7;3(2):vex036. doi: 10.1093/ve/vex036. eCollection 2017 Jul. Virus Evol. 2017. PMID: 29250433 Free PMC article.
References
- McDaniel LD, Young E, Delaney J, Ruhnau F, Ritchie KB, Paul JH. 2010. High frequency of horizontal gene transfer in the oceans. Science 330:50. - PubMed
- Lang AS, Beatty JT. 2007. Importance of widespread gene transfer agent genes in alpha-proteobacteria. Trends Microbiol. 15:54–62 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources